Extenuating the role of Ficus virens Ait and its novel bioactive compound on antioxidant defense system and oxidative damage in cigarette smoke exposed rats
Abstract
Introduction: Production of free radicals is associated with cigarette smoke (CS) which in turn generates oxidative stress, could be responsible for alterations in the activities of enzymatic and non-enzymatic antioxidants that links with atherosclerosis.
Methods: Therefore, the putative preventive effects of F. virens extract and its bioactive compound (F18), n-Octadecanyl-O-α-D-glucopyranosyl(6’→1’’)-O-α-D-glucopyranoside were investigated on overall enzyme and non-enzymatic defense system and in oxidative stress CS-exposed rats.
Results: The enzymatic activities of hepatic and lung CAT, SOD, Gred and GST in CS exposed rats were significantly decreased, while Gpx activity in CS exposed rats was increased. Similarly, hepatic and lung GSH content was reduced when compared to value of normal control group. Simultaneous administration of FVBM extract (50 and 100 mg/rat) and F18 bioactive compound (1 mg/rat) significantly increases hepatic and lung CAT, SOD, Gred and GST activity as well GSH concentration coupled with decrease in Gpx level in CS-exposed stress rats. Moreover, our histological observations concludes the pulmonary congestion, thickening of interalveolar septa and foci of collapsed alveoli with subsequent dilation of the adjoining alveolar spaces as well as development of large irregular spaces in rats lung exposed to cigarette smoke. Similarly, the liver also showed morphological alterations with congestion in central vein, portal inflammation and necrosis in CS-exposed rats. These morphological changes reversed significantly after treatment with FVBM extract and F18 compound.
Conclusion: Thus biochemical and histopathological studies suggested that, FVBM extract and F18 showed its protective nature against CS-exposed rats.
Introduction
Oxidative stress is one of the major symptoms accompanying physiological functions and many pathological conditions such as cancer, diabetes, chronic obstructive pulmonary disease, cardiovascular and neurodegenerative diseases and also in the aging process itself Aruoma, 1998Santilli et al., 2015Sen et al., 2010. Cigarette smoking (CS) contributes a considerable amount of free radicals, estimated as 1014and 1015 free radicals/puff in the tar and gas phases Church and Pryor, 1985 and is the major risk factor as well as leading cause cardiovascular disease (CVD) Messner and Bernhard, 2014WHO, 2015.
Moreover, CS generate free radicals that could be responsible for alterations in the activities of enzymatic viz., superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (Gpx), glutathione reductase (Gred), glutathione-s-transferase (GST) and non-enzymatic, namely GSH, antioxidants Ramesh et al., 2007Ramesh et al., 2015Thirumalai et al., 2011.
Various studies suggested that medicinal plants are source for the prevention of numerous oxidative stress related diseases Akhter et al., 2013Alvi et al., 2015Khan et al., 2013Salvamani et al., 2014. Thus, natural compounds with antioxidant properties could contribute to the protection of cell and tissue against deleterious effects caused by CS generated reactive oxygen species (ROS). Ficus species exhibits strong antioxidant and biological properties, known to diffuse the toxic free radical and can be used as a possible protective agent for treatment of oxidative stress related disorders Iqbal et al., 2014aSirisha et al., 2010T S et al., 2013. We have previously shown that Ficus virens bark methanolic (FVBM) extract contained large amount of antioxidant with significant hypolipidemic property Iqbal et al., 2014bIqbal et al.,2015. The current investigation demonstrates the protective role of FVBM extract and its principal bioactive compound, n-Octadecanyl-O-α-D-glucopyranosyl( 6’→1’’)-O-α-D-glucopyranoside in cigarette smoke-induced oxidative stress.
Materials and Methods
Chemical Reagents
Bradford dye was purchased from Sigma Aldrich, India, potassium dichromate, hydrogen peroxide (H2O2), glacial acetic acid was procured from Merck Pvt Ltd, India; capston cigarette from Capston, India ltd. All other chemicals were procured either from Himedia Laboratories, Mumbai, India or of analytical grade.
Isolation of Bioactive compound
Bioactive compound; n-Octadecanyl-O-α-Dglucopyranosyl (6→1″)-O-α-D-glucopyranoside (F18) from FVBM extract was isolated by following the protocol Iqbal et al., 2015.
Animals
Male Sprague-Dawley (SD) rats weighed around 100- 150 gm were procured from Indian Institute of Toxicology Research Center, Lucknow. The proposed study was approved by Institutional Animal Ethics Committee (IAEC) (registration number: IU/Biotech/project/CPCSEA/13/11). The rats were housed 5 per cage for one week in the animal house for acclimatization at a temperature of 21-22°C with 12 hours light and dark cycle. The rats were given standard diet and water ad libitum.
Dose preparation
Sequentially extracted FVBM extract, its bioactive fraction (F18) and reference drug atorvastatin were dissolved in 10 % dimethyl sulfoxide (DMSO) at different concentrations and were homogenized with saline. The doses of the extracts were selected on the basis of previously published reports Iqbal et al., 2015T S et al.,2013.
Diet/exposure to cigarette smoke
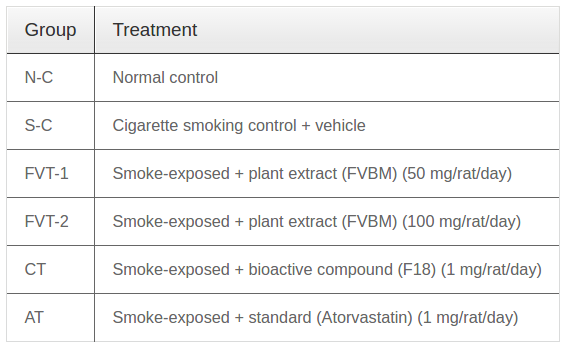
FVBM extract, its bioactive fraction (F18) and atorvastatin suspension was administered through gastric intubation in two divided doses (morning and evening) of 0.5 ml each/rat/day. Rats in smoking control group received 0.5 ml of saline containing 10 % DMSO (vehicle) twice daily while rats in normal control group received 0.5 ml of saline containing 10 % DMSO twice daily. The rats were divided randomly and equally (5 rats in each group) in groups as illustrated in Table 1 .
Rats were exposed to cigarette smoke in the morning by keeping two rats in bottomless metallic container (10 x 11 x 16 inch), having two holes of 3 and 1.5 cm diameter, one on the either side. A burning cigarette was introduced through one hole (3 cm) and the other hole (1.5 cm) was used for ventilation. Animals were exposed to CS for 30 minutes, daily for 4 weeks with interval of 10 min between each 10 min exposure, using 3 cigarettes/day/2 rats in each group Anbarasi et al., 2006.
Collection of different organs
Liver and lung were excised and kept in ice-cold saline. A portion of liver and lung were immediately fixed in 10 % neutral formalin for histopathological studies.
Preparation of homogenate and post mitochondrial supernatant
At the end of the experiment, liver and lung from the rats were promptly excised and chilled in ice-cold saline. After washing with saline, it was blotted and weighed. One gram of wet tissues was cut into pieces and homogenized with 9 ml of chilled 0.1 M sodium phosphate buffer, pH 7.4 (containing 1.17 % KCl) in a waring blender. The homogenate was centrifuged at 1,000 rpm for 10 min at 4°C, aliquoted and stored at -20°C. The remaining portion of the liver and lung homogenates was centrifuged at 12, 000 rpm for 20 min at 4°C. The post mitochondrial supernatant (PMS) thus obtained was also aliquoted and stored at –20°C for future use.
Activities of antioxidant enzymes
The enzymatic activity of catalase in PMS of liver and lung was measured by adopting the procedure of Sinha (1972). Enzymatic activity of SOD in PMS fraction of liver and lung was determined by the method as described by Kakkar et al. (1984) based on the 50 % inhibition of the formation of nicotinamide adenine dinucleotide (NADH)-phenazine methosulphatenitroblue tetrazolium formazan at 560 nm. Glutathione peroxidase activity in liver and lung homogenate was assayed by modifying of the previous protocols Hafeman et al., 1974Mills, 1959. The enzymatic activity of Gred in liver and lung was determined according to the method of Carlberg and Mannervik Eriksson et al., 1975. Method of Habig et al. (1974) was used to measure the GST activity in PMS fraction of liver and lung.
Activity of non-enzymatic antioxidant
For the determination of GSH content in liver or lung homogenate, the previous methods were followed Ellman, 1959Sedlak and Lindsay, 1968.
Histopathological studies of liver and lung
For histopathological study, a portion of liver and lung were used. For microscopic preparation of the above tissues, method of Disbrey and Rach (1970) was used. Two formalin fixed samples from each tissue were embedded in paraffin and sectioned after block preparation. The paraffin block was sectioned in 4~5 μm thickness by using a microtome to make a slide and then it was dyed with hematoxylin-eosin (H&E) and observed under a light microscope and photographs were taken JA, 2001 .
Protein estimation
The protein concentration of liver homogenate and PMS was analyzed by the method of Bradford (1976) using bovine serum albumin as standard. Aliquots of liver homogenates and PMS were first precipitated with 10 % TCA followed by centrifugation at 1500 rpm for 10 min. The protein pellets were dissolved in 0.5 N NaOH and used for protein determination.
Data analysis
For all assays, samples were analysed in triplicate and the results were expressed as mean ± SD. The results were evaluated using one-way analysis of variance (ANOVA) and two tailed Students T-test. Statistical significance were expressed as *p<0.05, **p<0.01 and ***p <0.001.
Results
Regulatory effects of FVBM extract, F18 bioactive compound and atorvastatin on antioxidant defense system in smoke-exposed rats after 4 weeks of treatment
Free radicals released by cigarette smoke (CS) generate oxidative stress. This process could be responsible for alterations in the activities of enzymatic and non-enzymatic antioxidants and development of atherosclerosis. Since, protection against oxidative stress/ROS is provided by enzymatic and non-enzymatic antioxidants, therefore, the status of antioxidant enzymes, such as CAT, SOD, Gpx, GST and Gred including GSH concentrations in liver and lung of experimental smoke-exposed rats are important.
Impact on the regulation of hepatic and lung catalase, superoxide dismutase, glutathione peroxidase, glutathione reductase, glutathione-s-transferase activities and reduced glutathione content
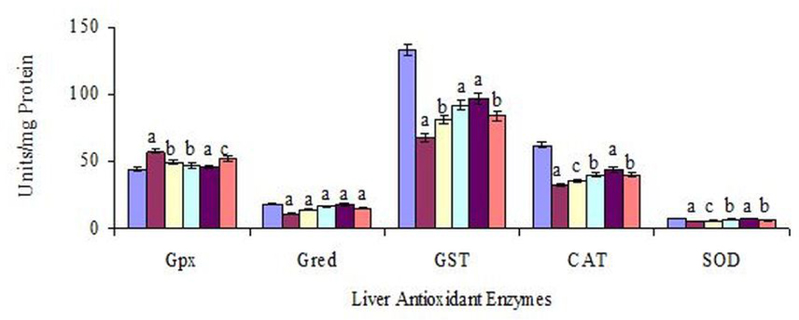
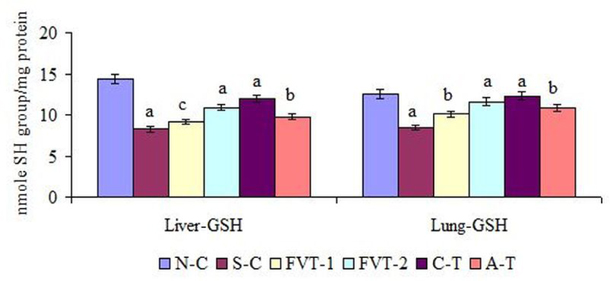
The enzymatic activities of hepatic CAT, SOD, Gred and GST in S-C rats were significantly decreased from N-C values of 62.28, 7.65, 18.29 and 132.8. U/mg protein to 32.45, 4.8, 11.28 and 68.12 U/mg protein, respectively, while Gpx activity in S-C rats was increased from N-C values of 44.2 to 57.28 U/mg protein ( Figure 1 ). The restoration in above enzymatic activities of hepatic CAT, SOD, Gred and GST in FVT-2 treated rat was 24, 32, 48 and 36 % of respective normal control values. The values of these enzymes in C-T group, in comparison to S-C values, were significantly increased by 35, 53, 60 and 43 % respectively. In contrast the hepatic Gpx activity was significantly decreased by 14, 18, 20 and 10 % in FVT- 1, FVT-2, C-T and A-T rats. As depicted in Figure 3 , the hepatic GSH content were reduced by 43 % when compared to value of N-C group. Administration of FVBM extract, F18 bioactive compound and atorvastatin to CS-exposed rats resulted in a significant increase in GSH content by 22, 32, 45 and 18 % respectively.
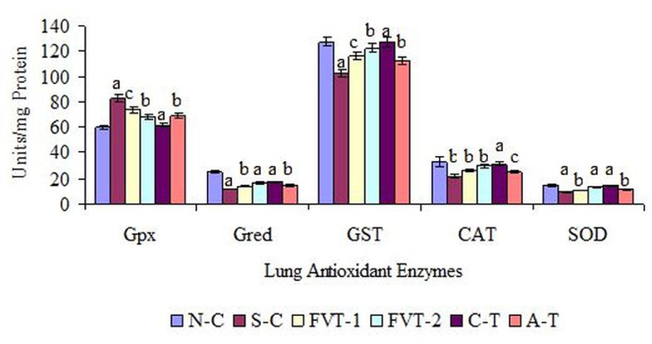
Moreover, similar pattern was observed in lung enzymatic and non-enzymatic antioxidant defense system. As depicted in Figure 2 the enzymatic activities of lung CAT, SOD, Gred and GST in S-C rats were significantly decreased from N-C value of 32.5, 14.7, 24.3 and 127.7 U/mg protein to 21.86, 9.56, 11.78 and 102.47 U/mg protein Figure 2 . Whereas, lung Gpx activity in SC rats was significantly increased from N-C values of 59.68 to 82.73 U/mg protein. The restoration in above enzymatic activities of lung CAT, SOD, Gred and GST in FVT-2 treated rat was 34, 40, 41 and 20 % of respective N-C values. The values of these enzymes in C-T group, in comparison to N-C values were significantly increased by 42, 50, 47 and 24 % respectively. In contrast the lung Gpx activity was significantly decreased by 11, 17, 26 and 16 % in FVT-1, FVT-2, C-T and A-T treated rats. Similarly, lung GSH content which was significantly reduced by 33 % in S-C rats as significantly increased by 20, 37, 46 and 28 % after administration of FVBM extract, F18 bioactive compound and atorvastatin Figure 3 . Here it is interesting to mention that atorvastatin also exhibited significant amelioration in all the enzymatic and non-enzymatic activities but is considered to be less effective than higher dose of FVBM extract and F18 bioactive compound.
In summary, hepatic and lung CAT, SOD, Gred, GST enzymes and non-enzymatic GSH, which constitute a mutually supportive team of defense against ROS, are significantly ameliorated after feeding of FVBM extract and F18 bioactive compound as well as substantially quenches the free radicals, thus positively normalizing the above enzyme levels close to normal values.
Histopathological studies of liver and lung
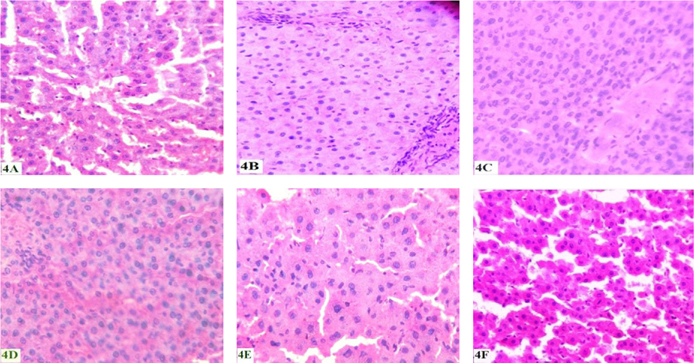
It is apparent from Figure 4A that histopathology of the liver of the control rats showed normal looking uniform hepatocytes with small vesicular nuclei and abundant eosinophilic granular cytoplasm with distinct cell boundaries. Architecture of liver is well maintained. Normal Interstitial cells with vascularity. CS-exposed rats showed smaller hepatocytes with small vesicular or pyknotic nuclei and reduced granularity of cytoplasm. Cytoplasmic boundaries are relatively indistinct. Architecture not well maintained. Interstitial cells increased and vascularity is reduced ( Figure 4B ). Administration of FVBM extract at lower dose has shown smaller hepatocytes with smaller vesicular nuclei and eosinophilic cytoplasm with indistinct cell boundaries showing proliferation. Architecture not well maintained but vascularity has relatively increased ( Figure 4C ). Moreover, liver section of FVT-2 rats (higher dose) ( Figure 4D ) showed proliferated normal hepatocytes with normal vesicular nuclei and abundant eosinophilic cytoplasm with distinct cell boundaries. Architecture well maintained with normal interstitial cell and vascularity. Similarly, liver section of CT rats showed proliferative hepatocytes with normal morphology as well as architecture. Interstitial cells normal with normal vascularity, also no toxic effects noted ( Figure 4E ). Furthermore, liver section of A-T rats showed normal hepatocytes with normal vesicular nuclei and abundant eosinophilic cytoplasm alongwith well maintained normal architecture and vascularity ( Figure 4F ).
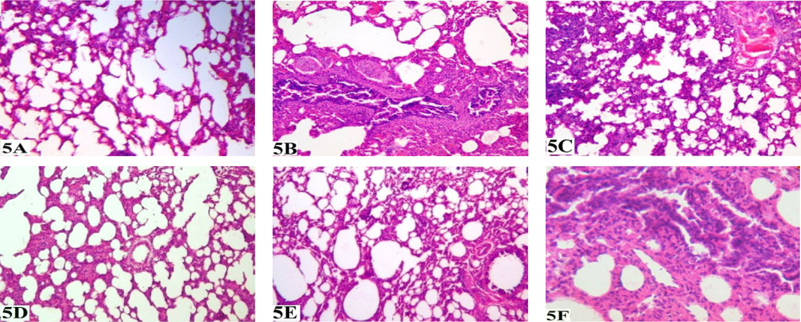
Histopathology of lung in N-C group shows normal lung stroma and characteristic spongy appearance of the lung with normal looking numerous alveolar spaces, had normal blood vessels and bronchi. The bronchoalveolar unit parenchyma in the normal lung was within the limits ( Figure 5A ). The lung section of CSexposed rats showed the increased volume of stroma with presence of few collections of stromal cells with reduced alveolar spaces, reduced vascularity and constricted bronchi ( Figure 5B ). There was obliteration of most alveoli and subsequently compensatory dilatation and expansion of the contiguous ones with destruction of alveolar wall. On treatment with lower dose of FVBM extract, section of lung shows reduced stroma, increased alveolar spaces, vascularity with patent blood vessels and dilated bronchi ( Figure 5C ). On the other hand, treatment with higher dose, section of lung showed changes as described above but bronchi are relatively constricted ( Figure 5D ). In addition, in lung section of C-T rat observed that stroma is further reduced with presence of large alveolar spaces. Blood vessels were normal but smaller bronchi were further constricted ( Figure 5E ). Furthermore, lung slice of atorvastatin treated group showed the alveolar spaces comparatively less than normal with corresponding increase in stromal elements. Blood vessels and bronchi were normal in the presence of few constricted smaller bronchi ( Figure 5F ).
Discussion
Cigarette smoking (CS) is the foremost cause of morbidity and mortality worldwide Alam et al., 2013Jha et al., 2008 and is considered to be the preventable risk factor for CVD Ambrose and Barua, 2004. Oxidative stress caused by CS are responsible for enhanced lipid peroxidation, protein oxidation, DNA damage and endothelial damage that could results in various diseases such as CVD, stroke,different types of cancer and diabetes Messner and Bernhard, 2014Mohod et al., 2014Valavanidis et al., 2009Willi et al., 2007.
Cigarette smoke is a complex situation possessing an array of free radicals and ROS Pryor and Stone, 1993. These, free radicals are highly reactive molecules occurs in normal consequence of a variety of biochemical reactions S, 2011. And their overload from exogenous sources like, smoking, alcohol abuse, UV radiations and air pollution added to the endogenous production of free radicals that results in oxidative stress and oxidative damage to the tissues Ghobashy et al., 2010Mohod et al., 2014Wu and Cederbaum, 2003 as well as to DNA, proteins and membrane lipids Cosmas Achudume and Aina, 2012Wu and Cederbaum, 2003.
Under oxidative stress conditions, the antioxidant enzyme levels are altered, in order to cope with the tremendous increase in the production of ROS Anbarasi et al., 2006McCord, 1993Mohod et al., 2014. However, after prolonged exposure, the toxic effects of cigarette smoke emerge to override the adaptive mechanism of the body tissues, as indicated by a decrement in the levels of these enzymes Hulea et al.,1994. It is clear from the above discussion CSinduces paramount oxidative stress and is consistent with our results that demonstrate significant decrease in liver and lung enzymatic activity of SOD, CAT, Gred, GST including the GSH level, while Gpx activity was significantly increased in CS-exposed rats.
In antioxidant defense system SOD is known to be the first enzyme, responsible for scavenging the superoxide radicals to form H2O2 Andersen et al., 1997. Furthermore, H2O2 is scavenged by catalase/GSH (glutathione peroxidase) or it facilitate in the development of highly reactive oxygen species In antioxidant defense system SOD is known to be the first enzyme, responsible for scavenging the superoxide radicals to form H2O2 Andersen et al., 1997. Furthermore, H2O2 is scavenged by catalase/GSH (glutathione peroxidase) or it facilitate in the development of highly reactive oxygen species Inoue et al., 2013Mohod et al., 2014. The tar phase of cigarette smoke contains quinone–semiquinone radicals which are capable of reducing molecular oxygen to superoxide radicals whose extreme generation inactivates this enzyme Church and Pryor, 1985Durak et al., 2002. The proposed decrease in SOD activity in CS-exposed rats could have resulted from its inactivation by tar phase oxidants. The presence and production of the free radicals from smoke lower enzymatic activity of CAT, leading to accumulation of H2O2 and lipid hydroperoxides which further deteriorate the tissue damage Pryor and Stone, 1993. Inhibition of CAT activity in rat liver and lung by cigarette smoke was observed during the present study which is in conjunction of previous reports Luchese et al., 2009Ramesh et al., 2007Ramesh et al., 2010.
However, a decrease in the activity of CAT in the present study suggests the in-vivodecrease in antioxidant level which in turn unable to defend the oxidative stress generated via cigarette smoke. Treatment with FVBM extract and F18 bioactive compound to smoke-exposed rats resulted in a significant increase in both liver and lung SOD and CAT activity which further strengthen the potent free radical scavenging property of plant extract and bioactive compound. Reduced glutathione play an important role in glutathione-dependent antioxidant system that most probably act as free radicals scavenger or a substrate for Gpx and GST during the detoxification of H2O2 Masella et al., 2005. Glutathione is maintained in body from its oxidized form by the enzyme Gred, which requires NADPH as a cofactor Carmel-Harel and Storz,2000. Previously it has been reported that GSH was depleted during CS-exposure in various tissues. Meanwhile, the decrease in tissue GSH levels in CS-exposed rats may be because of declined Gred activity and probably reduced NADPH supply Masella et al., 2005. GST is involved in the detoxification of ROS and toxic compounds from cigarette smoke, by conjugating them to GSH. This conjugation reaction results in depletion of the intracellular GSH that may further enhances oxidant injury probably due to nonavailability of GSH for antioxidant enzymes such as Gpx. Recent experimental data support the assumption that CS exposure increases oxidative stress and act as a potential mechanism for initiating cardiovascular dysfunction Ambrose and Barua, 2004.
Our data is well in agreement with above discussed reports and observed the significant decline in GSH, Gred and GST level in contrast the Gpx activity in liver and lung of CS-exposed rats was significantly increased which is due to inability of CAT to cope with the oxidative stress. Helen and Vijayammal (1997) also observed a similar decrease in the activity of SOD, CAT, Gred and an increase in Gpx in rats exposed to cigarette smoke. Gpx is also a scavenging enzyme, but an increase in its activity in tissues of CSexposed rats may further reduce the GSH content. In addition, an increased Gpx activity represents a compensatory mechanism to degrade H2O2. Simultaneous administration of FVBM extract (50 and 100 mg/rat) and F18 bioactive compound (1 mg/rat) significantly increases CAT, SOD, Gred and GST activity as well GSH concentration coupled with decrease in Gpx level in CS-exposed stress rats. Thus, it has been concluded that administration of FVBM extract at higher dose and F18 bioactive compound to CS-exposed rats restore the enzymatic (SOD, CAT, Gred, GST and Gpx) and non-enzymatic (GSH) antioxidant defense system and thus protect liver and lung cells against oxidative stress-induced damage by directly counteracting ROS/free radicals and by activating the overall antioxidant defense systems.
Histological observations noticed the pulmonary congestion and thickening of interalveolar septa in rats exposed to cigarette smoke. The present study showed devastation of some alveolar walls and foci of collapsed alveoli with subsequent dilation of the adjoining alveolar spaces and development of large irregular spaces (emphysematous changes). Cigarette smoking causes endothelial dysfunction due to increased oxidative stress Messner and Bernhard, 2014. Our results coincided with those who interrelated the thickening of the alveolar septa to modification in the vascular bed resulting in inflammatory infiltration and oedema Hora et al., 2003. Chronic cigarette smoke exposure causes lung tissue destruction might be due to increased production of metalloproteinases (MMP) proteolytic enzymes by macrophages Barnes et al., 2003Taraseviciene-Stewart and Voelkel, 2008. The liver showed congestion in central vein, portal inflammation and necrosis in CS-exposed rats. These morphological changes were significantly reversed after treatment with FVBM extract and F18 compound. Thus, biochemical and histopathological studies suggested that, FVBM extract and F18 showed its protective nature against CS-exposed rats.
Conclusion
In conclusion, our combined results clearly demonstrated the protective role of FVBM extract and F18 compound in CS-induced severe oxidative stress. The results are well supported by histopathological observations and indicates that F18/FVBM extract are highly promising natural antioxidant as well as can be used as an antioxidant and antiatherogenic agent. However, further large-scale clinical trials in smokers with and without coronary heart disease are required to substantiate their antioxidative, atheroprotective properties.
References
-
F.
Akhter,
A.
Hashim,
M.S.
Khan,
S.
Ahmad,
D.
Iqbal,
A.K.
Srivastava,
M.H.
Siddiqui.
Antioxidant, α- amylase inhibitory and oxidative DNA damage protective property of Boerhaavia diffusa (Linn.) root. South African Journal of Botany.
2013;
88
:
265-272
.
-
D.S.
Alam,
P.
Jha,
C.
Ramasundarahettige,
P.K.
Streatfield,
L.W.
Niessen,
M.A.H.
Chowdhury,
A.T.
Siddiquee,
S.
Ahmed,
T.G.
Evans.
Smoking-attributable mortality in Bangladesh: proportional mortality study. Bulletin of the World Health Organization.
2013;
91
:
757-764
.
-
S.S.
Alvi,
D.
Iqbal,
S.
Ahmad,
M.S.
Khan.
Molecular rationale delineating the role of lycopene as a potent HMG-CoA reductase inhibitor: in vitro and in silico study. Natural Product Research.
2015;
:
1-4
.
-
J.A.
Ambrose,
R.S.
Barua.
The pathophysiology of cigarette smoking and cardiovascular disease. Journal of the American College of Cardiology.
2004;
43
:
1731-1737
.
-
K.
Anbarasi,
G.
Vani,
K.
Balakrishna,
C.S.S.
Devi.
Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sciences.
2006;
78
:
1378-1384
.
-
H.R.
Andersen,
J.B.
Nielsen,
F.
Nielsen,
P.
Grandjean.
Antioxidative enzyme activities in human erythrocytes. Clinical chemistry.
1997;
43
:
562-568
.
-
O.I.
Aruoma.
Free radicals, oxidative stress, and antioxidants in human health and disease. Journal of the American Oil Chemists’ Society.
1998;
75
:
199-212
.
-
P.J.
Barnes,
S.D.
Shapiro,
R.A.
Pauwels.
Chronic obstructive pulmonary disease: molecular and cellularmechanisms. Eur Respir J.
2003;
22
:
672-688
.
-
O.
Carmel-Harel,
G.
Storz.
Roles of the Glutathione- and Thioredoxin-Dependent Reduction Systems in theEscherichia ColiandSaccharomyces CerevisiaeResponses to Oxidative Stress. Annual Review of Microbiology.
2000;
54
:
439-461
.
-
D.F.
Church,
W.A.
Pryor.
Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect.
1985;
64
:
111-126
.
-
A.
Cosmas Achudume,
F.
Aina.
Oxidative Stress and Oxidative Damage in Male Rat Erythrocytes Associated with Prolonged Exposure to Smoke Pollution. Journal of Environmental Protection.
2012;
03
:
414-419
.
-
İ.
Durak,
S.
ElgüN,
N.K.
BingöL,
M.Y.
Burak ÇImen,
M.
KaçMaz,
S.
BüYüKkoçAk,
H.S.
ÖZtüRk.
Effects of cigarette smoking with different tar content on erythrocyte oxidant/antioxidant status. Addiction Biology.
2002;
7
:
255-258
.
-
G.L.
Ellman.
Tissue sulfhydryl groups. Archives of Biochemistry and.
1959;
Biophysics82
:
70-77
.
-
S.
Eriksson,
P.E.R.
Askeloef,
K.
Axelsson,
I.
Carlberg,
C.
Guthenberg,
B.
Mannervik.
ChemInform Abstract: Resolution of glutathione-linked enzymes in rat liver and evaluation of their contribution to disufide reduction via thioldisulfide interchange. hemischer Informationsdienst.
1975;
6
.
-
H.A.
Ghobashy,
U.A.G.
Elmeleegy,
H.S.
Seleem.
Histological, Histochemical, Immunohistochemical and Morphometric Study of Adult Male Albino Ratʼs Lung Following Exposure to Air Pollution. The Egyptian Journal of Histology.
2010;
33
:
140-155
.
-
D.
Hafeman,
R.
Sunde,
W.
Hoekstra.
Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. The Journal of nutrition.
1974;
104
:
580-587
.
-
K.
Hora,
A.
Fonseca,
S.
Valença,
R.
Santos,
L.
Porto.
Lung morphometry in rats treated with intraperitoneal nicotine. Acta Microscopica.
2003;
12
.
-
S.
Hulea,
R.
Olinescu,
S.
Nita,
D.
Crocnan,
F.
Kummerow.
Cigarette smoking causes biochemical changes in blood that are suggestive of oxidative stress: a case-control study. Journal of environmental.
1994;
pathology
:
toxicology and oncology: official organ of the International Society for Environmental Toxicology and Cancer 14, 173-180
.
-
M.
Inoue,
S.
Nagi,
E.
Chadeka,
F.
Mutungi,
M.
Osada-Oka,
K.
Ono,
T.
Oda,
M.
Tanaka,
Y.
Ozeki,
K.D.J.
Yombo.
Relationship between mycobacterium tuberculosis and hookworm infections among school children in Mbita, Kenya. J Trop.
2013;
Dis1
:
2
.
-
D.
Iqbal,
M.S.
Khan,
A.
Khan,
M.S.
Khan,
S.
Ahmad,
A.K.
Srivastava,
P.
Bagga.
In Vitro Screening for β- Hydroxy-β-methylglutaryl-CoA Reductase Inhibitory and Antioxidant Activity of Sequentially Extracted Fractions of Ficus palmata Forsk. BioMed Research International.
2014a;
2014
:
1-10
.
-
D.
Iqbal,
M.S.
Khan,
M.
Khan,
S.
Ahmad,
A.K.
Srivastava.
An In Vitro and Molecular Informatics Study to Evaluate the Antioxidative and β-hydroxy-β-methylglutaryl-CoA Reductase Inhibitory Property of Ficus virens Ait. Phytotherapy Research.
2014b;
28
:
899-908
.
-
D.
Iqbal,
M.S.
Khan,
M.S.
Khan,
S.
Ahmad,
M.S.
Hussain,
M.
Ali.
Bioactivity guided fractionation and hypolipidemic property of a novel HMG-CoA reductase inhibitor from Ficus virens Ait. Lipids Health Dis.
2015;
14
.
-
K.
JA.
Histological and Histochemical Methods: Theory and Practice. Publication City/Country: London: Hodder Arnold.
2001
.
-
P.
Jha,
B.
Jacob,
V.
Gajalakshmi,
P.C.
Gupta,
N.
Dhingra,
R.
Kumar,
D.N.
Sinha,
R.P.
Dikshit,
D.K.
Parida,
R.
Kamadod.
A Nationally Representative Case-Control Study of Smoking and Death in India. New England Journal of Medicine.
2008;
358
:
1137-1147
.
-
M.
Khan,
M.A.
Khan,
I.
Ansari,
J.
Arif.
Dietary phytochemicals as potent chemotherapeutic agents against breast cancer: Inhibition of NF-κB pathway via molecular interactions in rel homology domain of its precursor protein p105. harmacognosy Magazine.
2013;
9
:
51
.
-
C.
Luchese,
S.
Pinton,
C.W.
Nogueira.
Brain and lungs of rats are differently affected by cigarette smoke exposure: Antioxidant effect of an organoselenium compound. Pharmacological Research.
2009;
59
:
194-201
.
-
R.
Masella,
R.
Di Benedetto,
R.
Varì,
C.
Filesi,
C.
Giovannini.
Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. The Journal of Nutritional Biochemistry.
2005;
16
:
577-586
.
-
J.M.
McCord.
Human disease, free radicals, and the oxidant/antioxidant balance. linical Biochemistry.
1993;
26
:
351-357
.
-
B.
Messner,
D.
Bernhard.
Smoking and Cardiovascular Disease: Mechanisms of Endothelial Dysfunction and Early Atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology.
2014;
34
:
509-515
.
-
G.C.
Mills.
The purification and properties of glutathione peroxidase of erythrocytes. Journal of Biological Chemistry.
1959;
234
:
502-506
.
-
K.
Mohod,
A.
Ninghot,
A.K.
Ansari,
N.
Garg.
Circulating Lipid Peroxide and Antioxidant Status in Cigarette Smokers: An Oxidative Damage Phenomena. International Journal of Health Sciences and Research (IJHSR).
2014;
4
:
59-65
.
-
W.A.
Pryor,
K.
Stone.
Oxidants in Cigarette Smoke Radicals, Hydrogen Peroxide, Peroxynitrate, and Peroxynitrite. Ann NY Acad Sci.
1993;
686
:
12-27
.
-
T.
Ramesh,
R.
Mahesh,
V.H.
Begum.
Effect of Sesbania grandiflora on Lung Antioxidant Defense System in Cigarette Smoke Exposed Rats. International Journal of Biological Chemistry.
2007;
1
:
141-148
.
-
T.
Ramesh,
C.
Sureka,
S.
Bhuvana,
V.H.
Begum.
Brain oxidative damage restored by Sesbania grandiflora in cigarette smoke-exposed rats. Metabolic Brain Disease.
2015;
30
:
959-968
.
-
T.
Ramesh,
C.
Sureka,
S.
Bhuvana,
V.
Hazeena Begum.
Sesbania grandiflora diminishes oxidative stress and ameliorates antioxidant capacity in liver and kidney of rats exposed to cigarette smoke. Journal of Physiology and Pharmacology.
2010;
61
:
467
.
-
V.
S.
Free radicals in health and diseases. Pharmacol online.
2011;
1
:
1062-1077
.
-
S.
Salvamani,
B.
Gunasekaran,
N.A.
Shaharuddin,
S.A.
Ahmad,
M.Y.
Shukor.
Antiartherosclerotic Effects of Plant Flavonoids. BioMed Research.
2014;
International2014
:
1-11
.
-
F.
Santilli,
M.T.
Guagnano,
N.
Vazzana,
S.
Barba,
G.
Davi.
Oxidative Stress Drivers and Modulators in Obesity and Cardiovascular Disease: From Biomarkers to Therapeutic Approach. CMC.
2015;
22
:
582-595
.
-
J.
Sedlak,
R.H.
Lindsay.
Estimation of total, proteinbound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Analytical Biochemistry.
1968;
25
:
192-205
.
-
S.
Sen,
R.
Chakraborty,
C.
Sridhar,
Y.
Reddy,
B.
De.
Free radicals, antioxidants, diseases and phytomedicines: current status and future prospect. nternational Journal of Pharmaceutical Sciences Review and Research.
2010;
3
:
91-100
.
-
N.
Sirisha,
M.
Sreenivasulu,
K.
Sangeeta,
C.M.
Chetty.
Antioxidant properties of Ficus species–a review. Int J PharmTech Res.
2010;
2
:
2174-2182
.
-
L.
T S,
S.
N B,
D.
S M,
J.
Pattar,
S.
K.
Repeated dose 28-day oral toxicity study of raw areca nut extract in rats. Int Res J Pharm.
2013;
4
:
238-240
.
-
L.
Taraseviciene-Stewart,
N.F.
Voelkel.
Molecular pathogenesis of emphysema. Journal of Clinical Investigation.
2008;
118
:
394-402
.
-
T.
Thirumalai,
E.
David,
S.V.
Therasa,
E.K.
Elumalai.
Restorative effect of Eclipta alba in CCl4 induced hepatotoxicity in male albino rats. Asian Pacific Journal of Tropical Disease.
2011;
1
:
304-307
.
-
A.
Valavanidis,
T.
Vlachogianni,
K.
Fiotakis.
Tobacco Smoke: Involvement of Reactive Oxygen Species and Stable Free Radicals in Mechanisms of Oxidative Damage, Carcinogenesis and Synergistic Effects with Other Respirable Particles. nternational Journal of Environmental Research and Public Health.
2009;
6
:
445-462
.
-
WHO.
World Health Organization (WHO) Cardiovascular diseases. In Fact Sheet No 317. Available at: http://www.who.int/mediacentre/factsheets/fs317/en/. Updated January, 2015.
2015
.
-
C.
Willi,
P.
Bodenmann,
W.A.
Ghali,
P.D.
Faris,
J.
Cornuz.
Active Smoking and the Risk of Type 2 Diabetes. JAMA.
2007;
298
:
2654
.
-
D.
Wu,
A.I.
Cederbaum.
Alcohol, oxidative stress, and free radical damage. lcohol Research and Health.
2003;
27
:
277-284
.
Comments

Downloads
Article Details
Volume & Issue : Vol 3 No 07 (2016)
Page No.: 723-732
Published on: 2016-07-26
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6702 times
- Download PDF downloaded - 1857 times
- View Article downloaded - 11 times
 Biomedpress
Biomedpress