Umbilical cord derived stem cell (ModulatistTM) transplantation for severe chronic obstructive pulmonary disease: a report of two cases
Abstract
Introduction: Chronic obstructive pulmonary disease (COPD) is a chronic disease affecting the airway of the respiratory system. COPD cases have rapidly increased in recent years, with the disease becoming the fourth leading cause of death worldwide. Stem cell transplantation is a new approach to treat COPD. In this study we report in two cases the use of transplanted stem cells to treat COPD.
Methods: Umbilical cord derived stem cells (ModulatistTM) were used in the study. ModulatistTM was prepared according to previous published studies. Two patients with late stage COPD (stage IV) were transfused with Modulatist at a dose of 106 cells/kg. Patients were evaluated by the COPD assessment test (CAT) score as well as the Modified Medical Research Council Dyspnea Scale (mMRC) score, before and after transplantation (1, 3 and 5 months post transplantation).
Results: Results showed that ModulatistTM transplantation significantly improved sever COPD, especially after 3 months. At that time point, the two patients receiving ModulatistTM showed a significantly improvement, from late-stage of COPD (stage IV) to stage I.
Conclusion: Although these initial results suggest that ModulatistTM transplantation is a promising therapy, more clinical studies in COPD patients are warranted to evaluate efficacy.
Introduction
COPD is the third-leading cause of death in the United States Kochanek et al., 2011 Minino, 2011 and fourth leading cause of death in the world. Therefore, many studies have been carried out to develop effective treatment methods. To date, development of treatments, such as pharmaceutical drugs, have led to reduction of symptoms. However, these treatments cannot attenuate disease progression or reverse COPD and emphysematous changes.
Increased interest in stem cells and their unique properties have led to investigations of stem cell transplantation as an alternative treatment for COPD. The most popular kind of stem cells for disease treatment has been mesenchymal stem cells (MSCs). MSCs can be isolated and cultured easily from various tissues in the human body, such as bone marrow and adipose tissue. However, procedures to collect MSCs from these tissues are invasive and laborious. Therefore, MSCs from umbilical cord blood, placenta or umbilical cord have emerged as alternative sources. Unlike other kinds of stem cells, MSCs exhibit unique properties; they secrete paracrine factors, play a role in immune modulation, and undergo multiple lineage differentiation. Given their immune modulatory properties, MSCs have been used effectively to treat various immune-related diseases.
MSCs have been investigated in mice for treatment of various lung diseases. These conditions include ventilator-induced lung injury (Curley et al., 2012), bleomycin-induced fibrosis Moodley et al., 2009 Ortiz et al., 2003, cigarette smoke-induced or elastase-induced COPD/emphysema Antunes et al., 2014 Chen et al., 2015 Zhao et al., 2014, bronchopulmonary dysplasia Aslam et al., 2009 Tropea et al., 2012, and bacterial pneumonia Gupta et al., 2012 Krasnodembskaya et al., 2012. A systemic and meta-analysis of 20 eligible preclinical studies using MSC transplantation for COPD treatment has shown that MSC administration significantly attenuates acute lung injury, stimulate lung tissue repair and improves lung function Liu et al., 2016. The mechanism of action mediated by MSC transplantation in COPD was also investigated and reviewed in that study; the most common mechanism was amelioration of airway inflammation Liu et al., 2016.
The promising preclinical studies have prompted clinical investigations to evaluate the application and efficacy of stem cells (particularly MSCs) in patients Shroff, 2015 Stessuk et al., 2013 Weiss et al., 2013. In the first study reported by Stessuk et al., the authors infused autologous bone marrow mononuclear cells into 4 patients with advanced pulmonary emphysema Stessuk et al., 2013. These patients were followed up for 3 years. The results showed that this procedure was safe for patients with chronic obstructive pulmonary disease, with no adverse effects recorded. Moreover, patients were showed improvement in clinical condition and quality of life Stessuk et al., 2013. In a subsequent study by Weiss et al., the authors performed a placebo-controlled, randomized trial using MSCs from bone marrow (Prochymal; provided by Osiris Therapeutics Inc.). Contrary to the hypothesis, the clinical trial showed that Prochymal cell transplantation showed low efficacy in COPD, despite significantly reduced serum C-reactive protein (C-RP) levels in the patients who received MSC administration Weiss et al., 2013. Recently, a case report using human embryonic stem cells to treat emphysematous COPD was reported Shroff, 2015. In this case, human embryonic stem cell transplantation resulted in improved symptoms of the emphysema patient Shroff, 2015. Our report herein represents 2 cases of patients with severe COPD who received treatment with umbilical cord derived stem cells (Modulatist™; provided by RegenMed Lab.). The safety and efficacy of transplantation were followed for 5 months.
Methods
Umbilical cord derived stem cells (Modulatist™) were isolated according to our published protocol Pham et al., 2016. All cryopreserved Modulatist™ cells were thawed and re-plated overnight to select for viable (adherent) cells. The next day, the adherent cells were detached and collected. Cell viability and cell number were analyzed by flow cytometer (Accuri C6; BD Biosciences, San Jose, CA). Only cell samples with greater than 95% cell viability were used for transplantation.
The patients were diagnosed with severe COPD (stage IV) based on forced expiratory volume in one second (FEV1). After transplantation with stem cells, patients were monitored and re-evaluated for their CAT score (i.e. COPD assessment test score), which is based on a validated test for evaluation of COPD impact on health status. Moreover, patients were evaluated on the Modified Medical Research Council Dyspnea Scale (mMRC), which uses a simple grading system to assess a patient's level of dyspnea, i.e. shortness of breath).
Case presentation
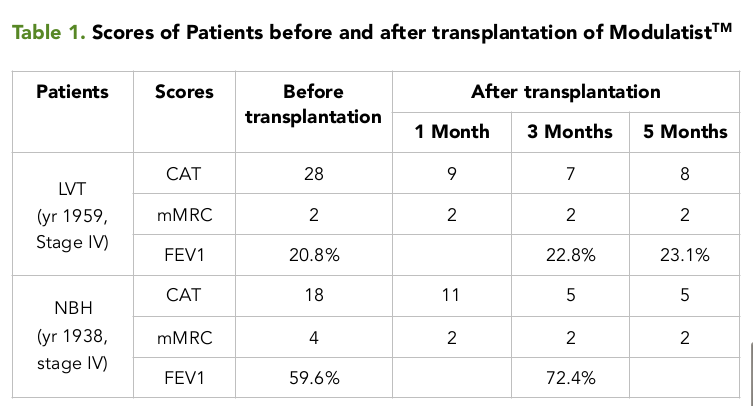
For the first patient (male; born in 1959) with stage IV COPD, evaluations included CAT, mMRC and FEV1. The results showed that FEV1 was low (at 20.8%), while CAT and mMRC scores were high (28 and 2, for CAT and mMRC, respectively). After diagnosis, the patient was transplanted with Modulatist™ at 106 cells/kg by transfusion into arm vein. All cells were prepared in 250 mL of sodium chloride (0.9%). The cell suspension was transfused in 30-45 minutes. Patients was monitored in the hospital for 1 week after transplantation for evaluation and recording of any side effects related to contamination. Following hospital discharge, the patient was monitored as an out-patient for 5 months after transplantation. The CAT and mMRC scores were evaluated after 1, 3 and 5 months. All traditional treatments were maintained and applied for the patient. The results showed the CAT score significantly decreased from 28 (before transplantation) to 9, 7 and 8 (at 1 month, 3 months and 5 months, respectively) Table 1 . However, the mMRC score stayed consistently at 2, before or after transplantation (1, 3 and 5 months). While the FEV1 slightly increased after Modulatist™ transplantation to 22.8% after 3 months and to 23.1% after 5 months, compared to 20.8% (before transplantation). The patient felt better and much healthier, and showed a significant reduction in acute exacerbation; the patient has been monitored for 12 months to date.
For the second patient (male; born in 1938) with stage IV of COPD, evaluations also included CAT, mMRC and FEV1 Table 1 . The results showed that FEV1 was low (at 59.6%), while CAT and mMRC scores were high (18 and 4, for CAT and mMRC, respectively). Similar to the first case, after diagnosis the patient was transplanted with Modulatist™ at 106 cells/kg by transfusion into arm vein. Likewise, all cells were prepared in 250 mL of sodium chloride (0.9%). The cell suspension was transfused in 30-45 minutes. Patient 2 was monitored in the hospital and out of the hospital, according to the same regimen and schedule as patient 1. The results showed that compared to the scores before transplantation, the CAT score significantly decreased 5 months post transplantation (from 18 to 5) and FEV1 increased 3 months post transplantation (from 59.6% to 72.4%). No complaints or side effects were noted for Modulatist™ during the 5-month monitoring post transplantation. Importantly, the number of hospital admissions related to exacerbations significantly reduced, from 13 admission per year to 0 admission during the 5 month follow-up.
Discussion
COPD is a prevalent and global disease, ranking worldwide as the fourth leading cause of mortality. Although there have been many medicines developed to treat this disease, the present-day medicines only reduce symptoms and pain. This study investigated the application of umbilical cord derived stem cells (called Modulatist™; produced by RegenMed Lab Ltd.) to treat 2 patients with severe COPD.
Although there were only 2 patients, and the monitoring was relatively short term, these results from these 2 patients are promising. The umbilical cord derived stem cells (Modulatist™ cells), produced by Modulatist™ technology, showed positive effects in the COPD patients. Firstly, during the 5 months after transplantation of Modulatist™, there were no recorded complications or side effects related to Modulatist™ in any of the patients. Secondly, with regard to treatment efficacy, administration of Modulatist reduced COPD symptoms, improved all scores (CAT, mMRC, and FEV1), and improved the patient’s quality of life. Particularly, the rate of acute exacerbation was significantly reduced in both patients.
These effects arise from properties and characteristics of Modulatist™ cells which have been published in our previous study Pham et al., 2016. For instance, Modulatist™ cells exhibit strong immune modulation, more so than adipose derived stem cells or bone marrow derived stem cells. As MSCs, Modulatist™ cells isolated from umbilical cord can inhibit T cells, B cells, and NK cells through various different mechanisms. Moreover, Modulatist™ cells can control inflammation as well as immune reactions inside the transplanted patients Pham et al., 2016. Given that inflammation is the main process contributing to COPD, the ability of the umbilical cord derived stem cells (Modulatist™) to modulate inflammation is highly beneficial.
COPD is a result of chronic inflammation at the airway of the respiratory system. Acute exacerbations of chronic obstructive pulmonary disease is characterized by increased pulmonary and systemic inflammation Tan et al., 2016. This process is triggered and increased by smoking or by air pollution. The long-term effects of inflammation leads to the obstructive condition, i.e. COPD. Moreover, the COPD attack considered to lead to the greatest risk of death is related to intense inflammation. With these reasons, Modulatist™ cells should be effective against COPD via immune modulation.
MSCs have shown some potential and success as treatment of diseases related to immune system, such as graft versus host disease (GVHD), autoimmune disease and liver cirrhosis. However, MSCs probably mediate their effects in different diseases via different mechanisms. Some mechanisms may include paracrine factors and in vivo differentiation of grafted cells. In animal models of COPD, MSCs have been shown to play a key role in stimulating lung tissue repair Liu et al., 2016. In a mouse model of COPD, it has been demonstrated that MSC transplantation can promote proliferation of endogenous lung stem cells Liu et al., 2015.
Conclusion
This study represents the first two cases of CODP patients treated with umbilical cord derived stem cells (Modulatist™). The transfusion of Modulatist™ into arm vein of late-stage COPD patients can significantly improve CAT and mMRC scores of the patients. Patient quality of life also improved with a significant reduction of acute exacerbation. Importantly, there were no adverse side effects recorded during the 5 month follow-up. Our results suggest that a clinical trial with more COPD patients needs to be evaluated to further confirm the safety and efficacy of umbilical cord derived stem cells (Modulatist™) transplantation in late-stage COPD treatment.
Lists of abbreviations
COPD: Chronic obstructive pulmonary disease; CAT: COPD assessment test; GVHD: graft versus host disease; mMRC: Modified Medical Research Council Dyspnea Scale; FEV1: forced expiratory volume in one second; MSC: Mesenchymal stem cell.
Ethics approval
This study was approved by Ministry of Health, Viet Nam. ClinicalTrials.gov Identifier: NCT02645305
Funding
This study used partly fund from Van Hanh Hospital and Laboratory of Stem Cell Research and Application, University of Science, VNU HCM, Viet Nam
Authors' contributions
PTBL and TMD: did clinical treatment, transfused stem cells into patients, acquired the data. NBV and PVP: prepared the stem cell products, did analysis and wrote the manuscript. All authors approved this manuscript.
References
-
M.A.
Antunes,
S.C.
Abreu,
F.F.
Cruz,
A.C.
Teixeira,
M.
Lopes-Pacheco,
E.
Bandeira,
P.C.
Olsen,
B.L.
Diaz,
C.M.
Takyia,
I.P.
Freitas.
Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir Res.
2014;
15
:
118
.
-
M.
Aslam,
R.
Baveja,
O.D.
Liang,
A.
Fernandez-Gonzalez,
C.
Lee,
S.A.
Mitsialis,
S.
Kourembanas.
Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med.
2009;
180
:
1122-1130
.
-
Y.B.
Chen,
Y.W.
Lan,
L.G.
Chen,
T.T.
Huang,
K.B.
Choo,
W.T.
Cheng,
H.S.
Lee,
K.Y.
Chong.
Mesenchymal stem cell-based HSP70 promoter-driven VEGFA induction by resveratrol alleviates elastase-induced emphysema in a mouse model. Cell Stress Chaperones.
2015;
20
:
979-989
.
-
N.
Gupta,
A.
Krasnodembskaya,
M.
Kapetanaki,
M.
Mouded,
X.
Tan,
V.
Serikov,
M.A.
Matthay.
Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax.
2012;
67
:
533-539
.
-
K.D.
Kochanek,
J.
Xu,
S.L.
Murphy,
A.M.
Minino,
H.C.
Kung.
Deaths: preliminary data for 2009. Natl Vital Stat Rep.
2011;
59
:
1-51
.
-
A.
Krasnodembskaya,
G.
Samarani,
Y.
Song,
H.
Zhuo,
X.
Su,
J.W.
Lee,
N.
Gupta,
M.
Petrini,
M.A.
Matthay.
Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol.
2012;
302
:
L1003-1013
.
-
H.M.
Liu,
L.J.
Ma,
J.Z.
Wu,
Y.G.
Li.
MSCs relieve lung injury of COPD mice through promoting proliferation of endogenous lung stem cells. J Huazhong Univ Sci Technolog Med Sci.
2015;
35
:
828-833
.
-
X.
Liu,
Q.
Fang,
H.
Kim.
Preclinical Studies of Mesenchymal Stem Cell (MSC) Administration in Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review and Meta-Analysis. PLoS One.
2016;
11
:
e0157099
.
-
A.M.
Minino.
Death in the United States, 2009. NCHS Data Brief.
2011;
:
1-8
.
-
Y.
Moodley,
D.
Atienza,
U.
Manuelpillai,
C.S.
Samuel,
J.
Tchongue,
S.
Ilancheran,
R.
Boyd,
A.
Trounson.
Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol.
2009;
175
:
303-313
.
-
L.A.
Ortiz,
F.
Gambelli,
C.
McBride,
D.
Gaupp,
M.
Baddoo,
N.
Kaminski,
D.G.
Phinney.
Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A.
2003;
100
:
8407-8411
.
-
P.V.
Pham,
N.B.
Vu,
N.K.
Phan.
Umbilical cord-derived stem cells (Modulatist™) show strong immunomodulation capacity compared to adipose tissue-derived or bone marrow-derived mesenchymal stem cells. Biomed Res Ther 3.
2016;
6
:
687-696
.
-
G.
Shroff.
Human embryonic stem cells (hESCs) in the treatment of emphysematous COPD: a case report. Clinical Case Reports.
2015;
3
:
632-634
.
-
T.
Stessuk,
M.A.
Ruiz,
O.T.
Greco,
A.
Bilaqui,
M.J.d.O.
Ribeiro-Paes,
J.T.
Ribeiro-Paes.
Phase I clinical trial of cell therapy in patients with advanced chronic obstructive pulmonary disease: follow-up of up to 3 years. Revista Brasileira de Hematologia e Hemoterapia.
2013;
35
:
352-357
.
-
D.B.
Tan,
N.E.
Ong,
M.
Zimmermann,
P.
Price,
Y.P.
Moodley.
An evaluation of CD39 as a novel immunoregulatory mechanism invoked by COPD. Hum Immunol.
2016;
77
:
916-920
.
-
K.A.
Tropea,
E.
Leder,
M.
Aslam,
A.N.
Lau,
D.M.
Raiser,
J.H.
Lee,
V.
Balasubramaniam,
L.E.
Fredenburgh,
S.
Alex Mitsialis,
S.
Kourembanas.
Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol.
2012;
302
:
L829-837
.
-
D.J.
Weiss,
R.
Casaburi,
R.
Flannery,
M.
LeRoux-Williams,
D.P.
Tashkin.
A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest.
2013;
143
:
1590-1598
.
-
Y.
Zhao,
A.
Xu,
Q.
Xu,
W.
Zhao,
D.
Li,
X.
Fang,
Y.
Ren.
Bone marrow mesenchymal stem cell transplantation for treatment of emphysemic rats. Int J Clin Exp Med.
2014;
7
:
968-972
.
Comments

Downloads
Article Details
Volume & Issue : Vol 3 No 10 (2016)
Page No.: 902-909
Published on: 2016-10-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5732 times
- Download PDF downloaded - 1776 times
- View Article downloaded - 38 times
 Biomedpress
Biomedpress