Establishment of a standardized mouse model of hepatic fibrosis for biomedical research
Abstract
Liver injury causes the formation of nodules and diffuses fibrosis or scar tissues termed liver fibrosis. Since efficient therapies to prevent fibrosis are not obtainable, it is necessary to build up a potential animal model of liver fibrosis to study. In this research, liver fibrosis micewere generated using Swiss mice and carbon tetrachloride - CCl4. CCl4 at doses of 0.8, 1.0 and 1.2 ml/kg was evaluated on mice to obtain an effective dose for liver fibrosis induction. This study aimed to build up a standardized hepatic fibrosis mouse model induced by carbon tetrachloride for further biomedical research. On Swiss mice, dose of CCL4 was considered and criterias of fibrosis were also evalutated such as serum markers, fibrosis marker-genes and histopathology. Mice were administrated with CCL4 in 8 consecutive weeks, 3 times/week. Body weight, survival rate and serum markers Aspartate aminotransferase/Alanine aminotransferase (AST/ALT), fibrosis markers (fibronectin, procollagen, nt5e, TGF-beta and integrin) and histopathology (Hematoxylin & Eosin staining) were measured to determine the suitable dose of CCL4. Results showed that CCL4 1.0 ml/kg is the efficient dose for liver fibrosis mouse model establishment. In a standardized liver fibrosis model, mice were treated with CCL4 1.0 ml/kg in 11 consecutive weeks, 3 times/week and evaluated serum markers level (AST, ALT, bilirubin, albumin), gene-expression of fibrosis markers using quantitative-RT PCR, histopathology (H&E staining) and connective tissue formation by Massive trichrome staining. The outcomes showed that serum markers and the level of fibrosis gene markers in standardized liver fibrosis mice had significantly increased. It is also recorded that there was a sharply increasing of fibronectin and procollagen expression (1222.40±4.20 and 241.35±1.18, respectively). We also recorded cirrhosis (fibrosis stage 3 – 5/6) in liver tissues of standardized liver fibrosis mice.
Introduction
Although there are many causes of chronic liver disease, such as liver cell injury (by alcohol, chemicals, viral hepatitis, etc.), bile duct injury, autoimmune disease/genetic disease, and metabolic dysfunction/disorder SJ, 2009, the consequences are the same. Liver injury causes nodule and scar tissue formation and diffuse fibrosis, characteristics encompassing liver cirrhosis, which leads to reduced liver function and increased risk of cancer SJ, 2009. End-stage liver disease (ESLD) is the final result of acute or chronic liver injury Heidelbaugh JJ, 2006. Moreover, 400 million people are infected with the hepatitis B virus worldwide Kapp, 2009. In Asian countries, the percentage of individuals infected with the hepatitis virus has been reported to be as high as 10% Li et al., 2012. ESLD is classified as the tenth leading cause of death, and 500,000–1,200,000 deaths occur each year because of the progression of viral infection to ESLD Kapp, Truong et al., 2014 2009.
Liver fibrosis is characterized by necrosis and inflammation, which increase numbers of Kupffer cells and activation of hepatic stellate cells, thereby contributing to the degeneration of liver tissues GI-PPEUM LEE, 2005. The liver normally exhibits limited cell turnover; however, as soon as cell loss or damage occurs, a regenerative process is rapidly induced, functioning to recover and maintain organ functions MR Alison,2009. This process then leads to enhancement of extracellular matrix (ECM) production and the proliferation of parenchymal and/or nonparenchymal cells, resulting in fibrogenesis and diffuse hepatic fibrosis. Because cirrhosis is the final stage of fibrosis, further studies are required to determine the molecular mechanisms of cirrhotic changes.
Regardless of the etiology of fibrosis, animal models of liver fibrosis are appropriate for studying hepatic fibrosis and cirrhosis. Although no model that specifically represent the etiologies of human liver cirrhosis have been developed, several animal models of human liver diseases are currently used, including models induced by hepatotoxins, carbon tetrachloride (CCl4) Domitrovic et al., 2009Ming-Ling Chang, 2005, 3,5- diethoxycarbonyl-1,4-dihydrocollidine (DDC) Ming-Ling Chang, 2005, silica, allyl alcohol (AA), alpha-naphthyl-isothiocyanate (ANIT), and bile duct ligation. These models have provided support for our understanding of the mechanisms of hepatic fibrosis and have enabled us to evaluate the safety and effectiveness of novel potential therapies for liver fibrosis and cirrhosis Ming-Ling Chang, 2005Starkel and Leclercq, 2011Yan Liu, 2013. Among hepatotoxins, CCl4 is often used to induce hepatic fibrosis and cirrhosis in animals because the underlying biochemical mechanisms and histological characteristics are similar to those observed in human liver cirrhosis Constandinou et al., 2005Fujii et al., 2010GI-PPEUM LEE, 2005Li et al., 2012. CYP2E1, an enzyme expressed in perivenular hepatocytes, metabolizes CCl4 into the CCl3+ radical, which causes centrilobular necrosis and alters the permeability of the plasma and mitochondrial membranes of hepatocytes Fujii et al., 2010. This induces inflammation and fibrogenesis and increases the generation of ECM, thereby triggering wound healing. Chronic CCl4 exposure results in formation of nodules and fibrosis, products of the wound healing process. Many studies have shown that longterm CCl4administration causes significant changes in histology Ming-Ling Chang, 2005Starkel and Leclercq, 2011. Specifically, CCl4 treatment has been shown to cause fibrosis after 2–4 weeks, significant bridging fibrosis after 5–7 weeks, cirrhosis after 9–11 weeks, and micronodular cirrhosis after 10–20 weeks Starkel and Leclercq, 2011. However, the dose and duration of CCl4 treatment required to induce such lesions depend on the species and strain of the animal model used, as well as the method (i.e., intraperitoneal, oral, or inhalation) and frequency of administration Ming-Ling Chang,2005Starkel and Leclercq, 2011. Therefore, we aimed to develop a mouse model of liver cirrhosis using induction by CCl4. This study mainly concentrated on analyzing the results of oral administration of CCl4 in Swiss mice, which are popular in Vietnamese laboratories.
Materials – methods
CCl4-induced liver fibrosis/cirrhosis in mice
This study was approved by our Institutional Ethical Committee (Laboratory of Stem Cell Research and Application). To determine the optimal dose of CCl4, healthy male Swiss mice were randomly divided into four groups (10 mice/group). Mice in groups I, II, and III were given 0.8, 1.0, or 1.2 mL/kg CCl4 (99.5% purity, UNI-CHEM Chemical Reagent, China), respectively, via oral administration three times per week for 8 consecutive weeks, while mice in the control group were treated with olive oil. At the end of the drug-treatment period, liver fibrosis was assessed based on the following criteria: body weight, survival rate (during the drug treatment period), liver function (serum aspartate aminotransferase [AST], alanine aminotransferase [ALT]), expression of fibrosis/cirrhosis-related genes, and histology (using hematoxylin and eosin [H&E] staining).
For the establishment of a standardized liver fibrosis model, 20 mice received CCl4 at the optimal dose determined as described above. Mice were treated with CCl4 for 11 consecutive weeks in order to induce hepatic cirrhosis Starkel and Leclercq, 2011. To analyze changes in the expression of fibrosis- related genes, such as fibronectin, integrin, nt5e, transforming growth factor-beta (TGF-β), and procollagen, quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) was conducted using GAPDH as a housekeeping gene and internal control. Combined H&E and Massive trichrome staining were used to evaluate the formation of connective tissues in the liver.
Liver function analysis (serum markers: AST, ALT, total bilirubin, and albumin)
Venous blood was collected into 1.5-mL tubes and then centrifuged at 3000 rpm for 10 min. Plasma was obtained, and the activities of AST and ALT (Diagnosticum Zrt., Hungary), as well as the levels of total bilirubin (QuantiChrom Bilirubin Assay Kit, Bioassay Systems, CA, USA) and albumin (QuantiChrom BCG Albumin Assay Kit, Bioassay Systems), were evaluated according to the manufacturer’s instructions.
Evaluation of the expression of fibrosis biomarkers
Mouse liver tissues were collected, and total RNA was extracted using Easy-BLUE Total RNA Extraction Kit (iNtON Biotechnology, Korea), according to the manufacturer’s instructions. For investigation of the optimal dose of CCl4, fibrotic gene expression was evaluated by RT-PCR. For the establishment of a standardized liver cirrhosis model, fibrotic gene expression was assessed by quantitative RT-PCR (Brilliant II QRT-PCR Master Mix Kit, 1-Step, Agilent, CA, USA) using primers specific for fibronectin (forward: TGAAGAGGGGCACATGCTGA; reverse: GTGGGAGTTGGGCTGACTCG), procollagen (forward: CCTGGACGCCATCAAGGTCTAC; reverse: CCAAGTTCCGGTGTGACTCG), integrin (forward: GCCAGGGCTGGTTATACAGA; reverse: TCACAATGGCACACAGGTTT), nt5e (CD73) (forward: TTTGGAAGGTGGATTTCCTG; reverse: CCTCTCAAATCCAGGGACAA), TGF-β (forward: CTTCAGCTCCACAGAGAAGAACTGC; reverse: CACAATCATGTTGGACAACTGCTCC), and GAPDH (forward: AAGTTGTCATGGATGACC; reverse: ATCACCATCTTCCAGGAGC).
Histopathology
Liver tissues were collected and fixed in 4% paraformaldehyde (Merck Millipore, Germany). H&E and Massive trichrome staining were performed according to the procedures of the Department of Pathology and Anatomy (University of Medicine and Pharmacy, HCM). The interpretation of results was based on the Knodell-Ishak histological activity index (Ishak Modified HAI).
Statistical analysis
Data analysis was conducted using GraphPad Prism 6 software and Microsoft Excel 2011.
Results
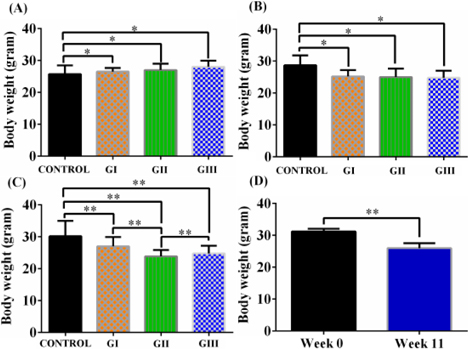
CCl4 (1.0 mL/kg) caused significant weight loss in mice after 8 weeks of treatment
At week 4, the body weights of mice in the CCl4 treatment groups were reduced compared to that of the control group. Mice in group II continued to gradually lose weight from week 0 to week 8 (from 27.91 ± 2.2 to 23.90 ± 2.51 g), and similar weight loss was recorded in group III. Meanwhile, mice in group I and the control group gained weight after 8 weeks of drug administration ( Figure 1A-C ).
All doses of CCl4 were associated with low mortality rates
After 8 weeks, dead mice were noted in all CCl4 treatment groups after 20 doses of CCl4 ( Figure 1D ). The highest survival rate was 85.71%, recorded in groups I and II. The death ratio increased significantly with the increase in CCl4 dose.
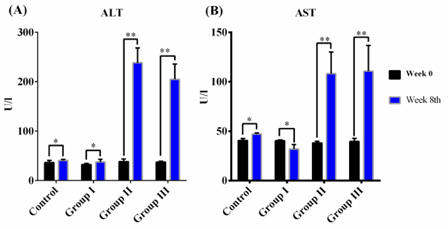
Serum markers of liver damage increased significantly in mice treated with 1.0 or 1.2 mL/mg CCl4
In the control group (treated with olive oil), serum AST and AST activities did not change after 8 weeks (p >0.05). In group I, serum AST levels did not change before, during, or after drug administration. However, serum AST and ALT levels were significantly increased after 8 weeks of CCl4 oral gavage in groups II and III. Serum AST increased by nearly 2.5-fold, while serum ALT rose nearly 5.0-fold after drug treatment ( Figure 2 ).
Changes in liver morphology after CCl4 treatment
After 8 weeks of CCl4 treatment, in the control group, the liver surface was glossy and had a bright red color. In contrast, in all treatment groups, there were multiple liver nodules, and the liver was slightly swollen, with a darkish discoloration throughout. These results supported that 8 weeks of CCl4 administration clearly altered the liver structure and health. The liver tissue surface was no longer smooth, but had multiple nodules and had a darkish color. This implied that CCl4 affected liver cells, causing liver damage and changes to the external morphology of the mouse liver.
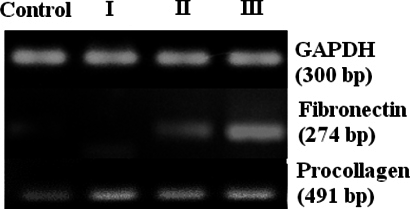
Expression of fibrogenesis- and ECM-related genes in the CCl4-treated group
RT-PCR was performed to assess the expression of fibrogenesis- and ECM-related genes. After 8 weeks of drug treatment, fibronectin was expressed in the livers of mice in groups II and III, but not in those of the control group and group I. Procollagen α1 expression was higher in CCl4- treated mice than in control mice ( Figure 3 ).
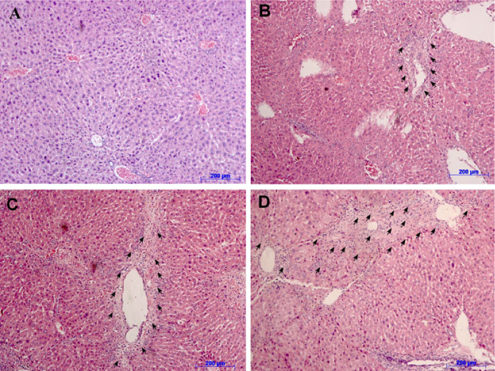
Histopathology was altered in CCl4-treated mice after 8 weeks of treatment
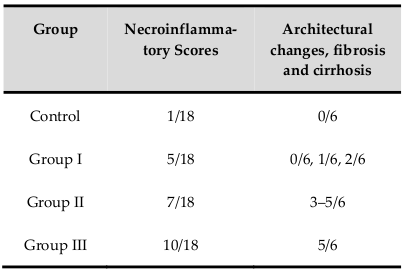
After 8 weeks, Hematoxylin and eosin staining results in all treatment groups showed different levels of liver fibrosis. Mice in the control group exhibited an inflammatory level of 1/18, without changes in the structures of blood vessels or bile ducts ( Figure 4A ). Lymphocytes were present, but only mild inflammation and no signs of fibrogenesis were observed in the control group.
However, all mice in the CCl4 treatment groups exhibited necrosis in some lobular areas and areas around the portal triad and central veins. Hematoxylin and eosin staining results showed fragmented nuclei, and lymphocytes and fibers were present ( Figure 4B-D ). These data supported that liver tissues were replaced by connective tissues. All liver samples from mice treated with CCl4 exhibited different levels of fibrosis, ranging from 3/6 to 5/6 according to the Knodell-Ishak index (Ishak Modified HAI) ( Table 1 ).
From these results, we chose a CCl4 dose of 1.0 mL/kg as the optimal dose for the standardized model since caused significant weight loss, low death rates, high levels of serum markers of liver damage, and marked changes in histopathology. Compared to other doses, 1.0 mL/kg CCl4 was the best dose for further experiments. For establishment of a standardized liver fibrosis mouse model, we extended the CCl4treatment time to cause liver cirrhosis.
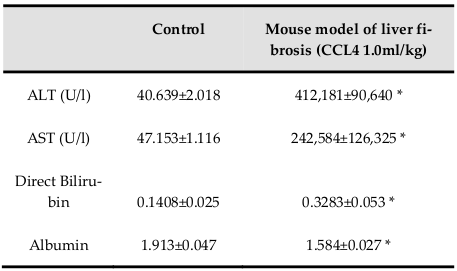
Body weights were significantly reduced during 11 weeks of treatment with 1.0 mL/kg CCl4 ( Figure 1D ). Increase of ALT and AST activities demonstrated the effectiveness of CCl4 for inducing liver toxicity. Additionally, levels of total bilirubin and albumin indicated the occurrence of liver dysfunction ( Table 2 ).
Changes in the expression levels of fibrosis markers in fibrosis model mice
The results showed that the gene expression levels of fibronectin, TGF-β1, integrin, nt5e, and procollagen were altered compared to those of the control ( Figure 5 ). Integrin levels increased dramatically in our mouse model of liver fibrosis. Moreover, we observed sharp increases in fibronectin and procollagen expression (1222.40 ± 4.20 and 241.35 ± 1.18, respectively). The levels of TGF-β1 and nt5e expression were slightly elevated compared with those in the control group.
Accumulation of fibers and connective tissue and changes in the histopathology of livers from our mouse model of hepatic fibrosis
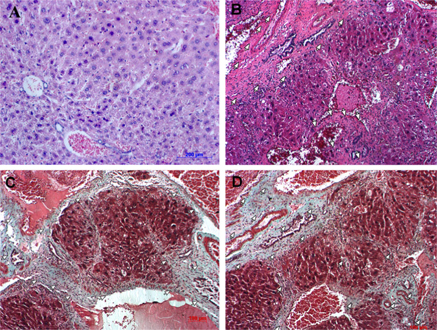
The microstructure of livers from mice treated with CCl4 differed significantly from that of normal livers from the control group ( Figure 6 ). Necrosis of hepatocytes was observed in the portal space and central lobular areas. Blood vessels and bile ducts exhibited accumulation of collagen fibers that gradually replaced healthy liver tissues. Collagen fibers occupied large areas of the liver, resulting in cytoplasmic shrinkage in hepatocytes and merging of cells, making it difficult to distinguish between cells. Fibrosis stages in these model mice were 3/6, 4/6, and 5/6. Massive trichrome staining also indicated that livers accumulated connective tissues, including collagen ( Figure 6 ). This illustrated that repetitive dosing of 1 mL/kg CCl4 (three times a week for 11 weeks) caused hepatic cirrhosis in Swiss mice.
Discussion
Because CCl4 is toxic and can cause mortality, establishment of a mouse model of liver fibrosis is challenging. According to our current study, gavage is suitable for CCl4administration as it resulted in low mortality compared to intraperitoneal administration of CCl4 Ming-Ling Chang, 2005. Moreover, estimating the degree of fibrosis in animal models is critical for determining whether therapeutic treatment could be effective. Traditionally, liver biopsy is considered the ‘gold standard’ for staging liver fibrosis. However, recent data have shown that there is a 30% failure/error rate in diagnoses by liver biopsy Mahato, 2007. Therefore, we used other techniques to accurately determine the liver fibrosis stage Mahato, 2007. To evaluate whether CCl4 can cause acute liver injury in Swiss mice, we relied on indirect markers, such as serum AST, ALT, total bilirubin, and albumin. Additionally, to confirm liver fibrosis, we evaluated fibrosis biomarkers/direct markers by qRT-PCR and classified liver histology based on the Knodell-Ishak (Ishak Modified HAI) scoring system.
Increases in ALT and AST levels may indicate the occurrence of liver necrosis Field et al., 2008. When liver cells are damaged, intracellular enzymes (including transaminase enzymes) leak into the blood and can be measured as indicators of cell necrosis. In our model, the level of serum ALT increased sharply in the context of hepatitis and acute liver injury. The results of AST and ALT levels in the control group were consistent with other reports Domitrovic et al., 2009. In group I, we observed insignificant changes in serum ALT and AST before and after CCl4 treatment, suggesting that injured liver cells were likely to be restored because of low CCl4 concentrations Mederacke, 2013. Moreover, increased AST and ALT levels in groups II and III were equivalent to those reported by Tsai et al (2009) Tsai et al., 2009. These results demonstrated that acute liver injury and inflammation occurred in our mouse model. The increases in AST and ALT levels in liver fibrosis induced by 1.0 mL/kg CCl4 supported that these mice exhibited substantial hepatic injury. A sharp increase in total bilirubin levels and a drop in albumin levels indicated loss of function in the mouse liver. These results were similar to those reported by Ohashi et al. (2012)Ohashi et al., 2012. Furthermore, our data showed that the ratio of AST/ALT in our mouse model was less than 1, supporting the occurrence of acute hepatitis Thapa and Walia, 2007. In order to confirm the presence of hepatic fibrosis, fibrosis markers and histopathology should also be evaluated.
Fibronectin and other proteins of the ECM create a framework for fibroblast migration. Fibronectin also provides direction and chemical signals for fibroblast proliferation Armbrust et al., 2004. Increased procollagen synthesis contributes to the increase in collagen levels observed in liver cirrhosis, and abnormal mRNA expression of procollagen α1 reflects the level of fiber in liver Du WD, 1999. In this study, we observed increased procollagen expression in CCl4-treated mice compared to that of normal mice. Because procollagen is a direct marker of fibrosis, abnormally high levels of procollagen indicate that liver tissues have accumulated scar nodules, accompanied by hepatitis and hepatic necrosis. In addition, high levels fibronectin Attallah et al.,2013, TGF-β1 Gressner et al., 2002, and integrin Stickel, 2011 expression may activate cells, such as fibroblasts and hematopoietic stem cells (HSCs). Nt5e has been shown to contribute to increased adenosine levels, which have an important role in hepatic fibrosis Peng et al., 2008. Indeed, synthetic ecto-5'-nucleotidase expression leads to increased levels of extracellular adenosine Peng et al., 2008. The increase in adenosine in injured liver tissue activates the cells through the A2A receptor, enhancing collagen synthesis and expression Che et al., 2007 and eventually leading to liver cirrhosis.
In this study, we applied the Ishak Modified HAI system to classify histological grading and staging of chronic hepatitis and fibrosis in mice. The results showed changes in hepatic histopathology, accumulation of ECM, and formation of nodules and fiber bridges. Our results of liver tissue histopathology were similar to the Massive trichrome staining results reported by Hao et al. (2012.
Conclusion
Based on the results of biochemical tests, anatomical observations, histological staining, and gene expression analysis, we concluded that oral administration of 1.0 mL/kg CCl4 for 11 weeks (three times per week, or once every two days) was sufficient for high-efficiency induction of liver fibrosis in Swiss mice. This mouse model of fibrosis had meaningfully changes in gene expression, as well as liver structure and function.
Abbreviations
AST aminotransferase; ALT Alanine aminotransferase; CCl4: carbon tetrachloride; CYP2E1: Cytochrome P450 2E1; ESLD: End stage liver disease; ECM: extracellular matrix; H&E: Hematoxylin and Eosin; Nt5e: ecto-5'-nucleotidase; TGFbeta: transforming growth factor beta
Authors' contributions
Truong Hai Nhung made substantial contributions to conception and design, acquisition of data and analysis and interpretation of data. Being corresponding author, Truong Hai Nhung gave final approval of the manuscript to be submitted and any revised version. Nguyen Hai Nam and Nguyen Thi Kim Nguyen who contributed to conduct some experiments, acquisition of data and participate in drafting the manuscript. Le Minh Huy and Tran Huong Giang made substantial contributions to analyze the histology change by using Knodell-Ishak (Ishak Modified HAI) scoring system. Huynh Nghia and Nguyen Van Thanh are supervisors of this study.
References
-
T.
Armbrust,
M.
Kreißig,
K.
Tron,
G.
Ramadori.
Modulation of fibronectin gene expression in inflammatory mononuclear phagocytes of rat liver after acute liver injury. Journal of Hepatology.
2004;
40
:
638-645
.
-
A.M.
Attallah,
A.A.
Abdallah So Fau - Attallah,
M.M.
Attallah Aa Fau - Omran,
K.
Omran Mm Fau - Farid,
W.A.
Farid K Fau - Nasif,
G.E.
Nasif Wa Fau - Shiha,
A.-A.F.
Shiha Ge Fau - Abdel-Aziz,
N.
Abdel-Aziz Aa Fau - Rasafy,
Y.M.
Rasafy N Fau - Shaker,
Y.M.
Shaker.
Diagnostic value of fibronectin discriminant score for predicting liver fibrosis stages in chronic hepatitis C virus patients. Ann Hepatol.
2013;
12
:
5344-5353
.
-
J.
Che,
E.S.
Chan,
B.N.
Cronstein.
Adenosine A2A receptor occupancy stimulates collagen expression by hepatic stellate cells via pathways involving protein kinase A, Src, and extracellular signal-regulated kinases 1/2 signaling cascade or p38 mitogen-activated protein kinase signaling pathway. Molecular pharmacology.
2007;
72
:
1626-1636
.
-
C.
Constandinou,
J.P.
Henderson N Fau - Iredale,
J.P.
Iredale.
Modeling liver fibrosis in rodents. Methods in Molecular.
2005;
Medicine
:
117, 237-250 117, 237
.
-
R.
Domitrovic,
H.
Jakovac,
J.
Tomac,
I.
Sain.
Liver fibrosis in mice induced by carbon tetrachloride and its reversion by luteolin. Toxicology and applied pharmacology.
2009;
241
:
311-321
.
-
Z.Y.
Du WD,
Zhou XM.
Zhai WR.
Dynamic changes of type I, III and IV collagen synthesis and distribution of collagenproducing cells in carbon tetrachloride-induced rat liver fibrosis. World journal of gastroenterology : WJG.
1999;
5
:
397-403
.
-
K.M.
Field,
M.
Dow C Fau - Michael,
M.
Michael.
Part I: Liver function in oncology: biochemistry and beyond. Lancet Oncol.
2008;
9
:
1092-1101
.
-
T.
Fujii,
B.C.
Fuchs,
S.
Yamada,
G.Y.
Lauwers,
Y.
Kulu,
J.M.
Goodwin,
M.
Lanuti,
K.K.
Tanabe.
Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC gastroenterology.
2010;
10
:
79
.
-
W.-I.J.
GI-PPEUM LEE,
SUN-HEE DO
DA-HEE JEONG.
Diagnostic Evaluation of Carbon Tetrachloride-induced Rat Hepatic Cirrhosis Model. ANTICANCER RESEARCH.
2005;
25
:
1029-1038
.
-
A.M.
Gressner,
K.
Weiskirchen R Fau - Breitkopf,
S.
Breitkopf K Fau - Dooley,
S.
Dooley.
Roles of TGF-beta in hepatic fibrosis. Front Biosci.
2002;
1
:
793-807
.
-
Z.M.
Hao,
M.
Cai,
Y.F.
Lv,
Y.H.
Huang,
H.H.
Li.
Oral administration of recombinant adeno-associated virus-mediated bone morphogenetic protein-7 suppresses CCl(4)-induced hepatic fibrosis in mice. Molecular therapy : the journal of the American Society of Gene Therapy.
2012;
20
:
2043-2051
.
-
B.M.
Heidelbaugh JJ.
Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician.
2006;
74
:
756-762
.
-
N.
Kapp.
WHO provider brief on hormonal contraception and liver disease. Contraception.
2009;
80
:
325-326
.
-
L.
Li,
Z.
Hu,
W.
Li,
M.
Hu,
J.
Ran,
P.
Chen,
Q.
Sun.
Establishment of a standardized liver fibrosis model with different pathological stages in rats. Gastroenterology research and practice.
2012;
2012
:
560345
.
-
K.C.a.R.I.
Mahato.
Gene Modulation for Treating Liver Fibrosis. Crit Rev Ther Drug Carrier Syst.
2007;
24
:
93-146
.
-
I.
Mederacke.
Liver fibrosis - mouse models and relevance in human liver diseases. Z Gastroenterol.
2013;
51
:
55-62
.
-
C.-T.Y.
Ming-Ling Chang,
Jeng-Chang Chen
Pei-Yeh Chang.
Comparison of murine cirrhosis models induced by hepatotoxin administration and common bile duct ligation. World Journal of Gastroenterology.
2005;
11
:
4167-4172
.
-
S.I.a.S.L .
MR Alison.
Stem cells in liver regeneration, fibrosis and cancer: the good, the bad and the ugly. Journal of Pathology.
2009;
217
:
282-298
.
-
K.
Ohashi,
Y.
Matsubara,
K.
Tatsumi,
A.
Kohori,
R.
Utoh,
H.
Kakidachi,
A.
Horii,
M.
Tsutsumi,
T.
Okano.
Cell Therapy Using Adipose-Derived Stem Cells for Chronic Liver Injury in Mice. Cell Medicine.
2012;
3
:
113-119
.
-
Z.
Peng,
P.
Fernandez,
T.
Wilder,
H.
Yee,
L.
Chiriboga,
E.S.
Chan,
B.N.
Cronstein.
Ecto-5'-nucleotidase (CD73) -mediated extracellular adenosine production plays a critical role in hepatic fibrosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology.
2008;
22
:
2263-2272
.
-
F.
SJ.
Stem Cell Therapy for Liver Disease. World Stem Cell Report (http://www.worldstemcellsummit.com ).
2009
.
-
P.
Starkel,
I.A.
Leclercq.
Animal models for the study of hepatic fibrosis. Best practice & research Clinical gastroenterology.
2011;
25
:
319-333
.
-
E.P.a.F.
Stickel.
Role of integrins in fibrosing liver diseases. Am J Physiol Gastrointest Liver Physiol.
2011;
301
:
G425-G434
.
-
B.R.
Thapa,
A.
Walia.
Liver function tests and their interpretation. Indian J Pediatr.
2007;
74
:
663-671
.
-
C.F.
Tsai,
W.-K.
Hsu Yw Fau - Chen,
W.-H.
Chen Wk Fau - Chang,
C.-C.
Chang Wh Fau - Yen,
Y.-C.
Yen Cc Fau - Ho,
F.-J.
Ho Yc Fau - Lu,
F.J.
Lu.
Hepatoprotective effect of electrolyzed reduced water against carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol.
2009;
47
:
2031
.
-
C.M.
Yan Liu,
Honglei Weng
Chengfu Xu.
Animal models of chronic liver diseases. Am J Physiol Gastrointest Liver Physiol.
2013;
304
:
G449-G468
.
Comments
Downloads
Article Details
Volume & Issue : Vol 1 No 02 (2014)
Page No.: 43-49
Published on: 2014-04-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 9155 times
- Download PDF downloaded - 2002 times
- View Article downloaded - 9 times
 Biomedpress
Biomedpress