Production of islet-like insulin-producing cell clusters in vitro from adipose-derived stem cells
Abstract
Diabetes mellitus is a high incidence disease that has increased rapidly in recent years. Many new therapies are being studied and developed in order to find an effective treatment. An ideal candidate is stem cell therapy. In this study, we investigated the differentiation of adipose derived stem cells (ADSCs) into pseudo-islets in defined medium in vitro, to produce large quantities of insulin-producing cells (IPCs) for transplantation. ADSCs isolated from adipose tissue were induced to differentiate into islet-like insulin-producing cell clusters in vitro by inducing medium DMEM/F12 containing nicotinamide, N2, B27, bFGF, and insulin-transferrin-selenite (ITS). Differentiated cells were analyzed for properties of IPCs, including storage of Zn2+ by dithizone staining, insulin production by ELISA and immunochemistry, and beta cell-related gene expression by reverse transcriptase PCR. The results showed that after 2 weeks of differentiation, the ADSCs aggregated into cell clusters, and after 4 weeks they formed islets, 50–400 micrometers in diameter. These islet cells exhibited characteristics of pancreatic beta cells as they were positive for dithizone staining, expressed insulin in vitro and C-peptide in the cytoplasm, and expressed pancreatic beta cell-specific genes, including Pdx-1, NeuroD, and Ngn3. These results demonstrate that ADSCs can be used to produce a large number of functional islets for research as well as application.
Introduction
Stem cells are one of the most exciting candidates for treating disorders and degenerative diseases such as diabetes mellitus. Presently, diabetes mellitus is treated by insulin injection therapy and Langerhan islet and beta cell transplantation. However, these therapies show a number of shortcomings, including sensing changes in the blood insulin level over time, development of insulin resistance, and the shortage of donor islets and cells. Stem cell therapy could address some of these shortcomings.
Adipose-derived stem cells (ADSCs) possess several attractive features, including availability, abundance, 500-fold higher frequency of mesenchymal stem cells (MSCs) and higher immunomodulatory capacity when compared to bone marrow-derived counterparts Melief et al., 2013. Moreover, they can differentiate into specific cells such as fat cells, osteocytes, chondrocytes and myocytes. Importantly, these cells of mesodermal origin can be driven to differentiate into endodermal cells such as insulin-producing cells via trans-differentiation Chandra et al., 2009Dave et al., 2013, 2014Fedyunina et al., 2011Hwang et al., 2011Marappagounder et al., 2013Mohamad Buang et al., 2012Moshtagh et al., 2013Timper et al., 2006. This study aimed to develop and test an efficient protocol to produce functional islets that can be used in research and application.
Material and methods
ADSC isolation and proliferation
Human adipose tissues were collected (with informed consent) from healthy women who underwent aesthetic surgery at the Van Hanh Hospital, Ho Chi Minh City, Vietnam. ADSCs were isolated using the ADSC Extraction Kit (GeneWorld Ltd. Co., Ho Chi Minh City, Vietnam) following the manufacturer’s instructions. Briefly, the obtained adipose tissue was washed three times with buffer solution to remove blood cells. The washed tissue was cut into many pieces and incubated with the enzyme mixture “SuperExtract” at 37°C for 30 min. The digested adipose tissue suspension was centrifuged at 3500 rpm for 10 min to obtain the stromal vascular fraction (SVF). Finally, these SVF cells were washed and cultured as previous published procedure at 37°C, 5% CO2 Van Pham et al., 2014. Medium was refreshed every 3 days. When the cell confluency reached 70–80%, cells were sub-cultured. ADSCs in the 3rd passage were used for further experiments.
In vitro differentiation of hADSCs into islet-like cell clusters (ICCs)
ADSCs were differentiated into ICCs by chemical induction. The differentiation process can be divided into three stages: (i) Stage 1: the cells were cultured in DMEM/F12 supplemented with 1% N2, 20% fetal bovine serum (FBS), and 1% antibiotic-antimycotic (all purchased from Sigma-Aldrich, St Louis, MO) for 10 days; (ii) Stage 2: the cells were cultured in differentiation medium containing DMEM/F12 supplemented with 2% FBS, 1% N2, 2% B27, b-FGF, and 10 mM nicotinamide (all purchased from Sigma-Aldrich) for 10 days; (iii) Stage 3: the cells were cultured in induction medium containing DMEM/F12 supplemented with 1% N2, 2% B27, b-FGF, 10 mM nicotinamide, 1 mM sodium pyruvate and insulintransferrin-selenium (ITS) (all purchased from Sigma-Aldrich) for 10 days. After 30 days, induced cells were analyzed for IPC properties.
ICC observation
After 30 days of induction, the number and size of ICCs were determined and counted under an inverted microscope using Axio Vision Microscopy Software (Carl-Zeiss, Germany).
Dithizone staining
ICCs were washed three times with phosphatebuffered saline (PBS) and fixed in 4% paraformaldehyde solution for 30 minutes. They were then stained with dithizone dye (DTZ). The dye was completely removed by washing the cells several times with buffer solution without Zn. Positive aggregated cells were observed by phase contrast microscopy (AxioVert 40C, Carl-Zeiss, Germany).
Immunofluorescence staining
The presence of insulin and C-peptide in the differentiated ICCs was evaluated using immunofluorescence staining. After 30 days of differentiation, ICCs were collected and rinsed several times with PBS and fixed in 4% paraformaldehyde solution. The fixed cells were permeabilized by 0.025% Triton X-100 (Merck, Germany), and blocked in 3.3% goat serum (Santa Cruz Biotechnology, Canada). Blocked cells were incubated with anti-C-peptide and anti-insulin primary antibodies (Santa Cruz Biotechnology, Canada) overnight at 4°C. The next day, the cells were stained with PE- or FITCconjugated secondary antibodies (Santa Cruz Biotechnology, Canada) for 1 hour. Finally, the cell nuclei were stained with 4’,6-diamidino-2- phenylindole (DAPI) (Santa Cruz Biotechnology, Canada) and observed by fluorescence microscopy (Cell Observer, Carl-Zeiss, Germany).
Insulin production measurement
The differentiated cells were stimulated by addition of glucose at concentrations of 25 mM, 35 mM, 45 mM, 55 mM, 65 mM and 75 mM. The glucose responsiveness of these cells were identified by insulin secretion. Insulin concentration was measured by ELISA. Briefly, glucose (Merck, Germany) and protease inhibitor (Sigma-Aldrich, St Louis, MO) were added to the differentiation medium at the 3rd stage. Differentiated cells were exposed to this medium for 24 hours, following which the supernatant was collected for insulin analysis. The supernatant was stored at 4oC until tested.
An insulin standard curve was developed from pure insulin (Sigma-Aldrich, St Louis, MO) diluted in 0.01 M hydrochloric acid. To determine the insulin concentration in the supernatant, the latter was added to 96-well ELISA plates (Santa Cruz Biotechnology, Canada) and incubated for 5 hours at room temperature. The plates were blocked with 5% bovine serum albumin (Sigma-Aldrich, St Louis, MO) overnight at 4oC, and then carefully washed twice with phosphate-buffered solution. Thereafter, the plates were stained with an anti-insulin primary antibody (Santa Cruz Biotechnology) for 3 hours at room temperature. After washing four times, the plates were incubated for 1 hour with secondary antirabbit IgG-HRP antibody (Santa Cruz Biotechnology). 3,3′,5,5′-Tetramethylbenzidine (TMB) liquid substrate was then added to each well and incubated for 30 minutes at room temperature, followed by the addition of 1 M H2SO4(stopping solution). Finally, the optical density (OD) was measured using a DTX 880 reader (Beckman Coulter) at wavelengths of 450 nm and reference 620 nm. All experiments were carried out three times independently.
Reverse transcription PCR
The total RNA of the ADSCs and differentiated cells were isolated by easy-BLUE™ total RNA extraction kit (iNtRON Biotechnology, Korea) according to the manufacturer’s instruction. The quality and quantity of RNA extraction was determined using a photometer (Eppendorf, Germany). Transcriptional gene expression was carried out using a ONE-STEP RT-PCR Premix kit (iNtRON) with specific primers for beta cell differentiation related genes ( Table 1 ). GAPDH was used as a housekeeping gene.
Reverse transcriptase polymerase chain reaction (RTPCR) was performed as a multi-step procedure: 30 minutes reverse transcription reaction at 45°C, 5 minutes RNA denaturation and cDNA hybridization at 94°C; followed by 35-cycles of 30 seconds denaturing at 95°C, 30 seconds annealing at 55-61°C ( Table 1 ), and 45 seconds elongation at 72°C; and a final elongation for 5 minutes at 72°C. PCR products were run on 2% agarose gels containing 0.5 μg/ml ethidium bromide and visualized using the UVP system (Upland, CA).
Statistical analysis
For the insulin standard curve, the data were analyzed and the graph was created by nonlinear regression (curve fit), sigmoidal, 4PL, X is log (concentration) (Prism 6, GraphPad Software, San Diego). The insulin levels of samples were interpolated from this standard curve. Other results were analyzed by one-way ANOVA. Data were reported as the mean and standard error of the mean (SEM). Statistical significance was defined as P < 0.05.
Results
ADSC isolation
ADSCs were determined to be mesenchymal stem cells as described by Dominici et al., 2006. In fact, they exhibited a fibroblast-like shape, were positive for MSC marker profiles CD44, CD73, CD90, CD105, and negative for blood cell makers CD14, CD34, CD45, and HLA-DR. They could also be differentiated into mesodermal origin cells such as osteoblasts and adipocytes. These results were presented by our group in the previous published study Van Pham et al., 2014.
Islet-like cluster formation
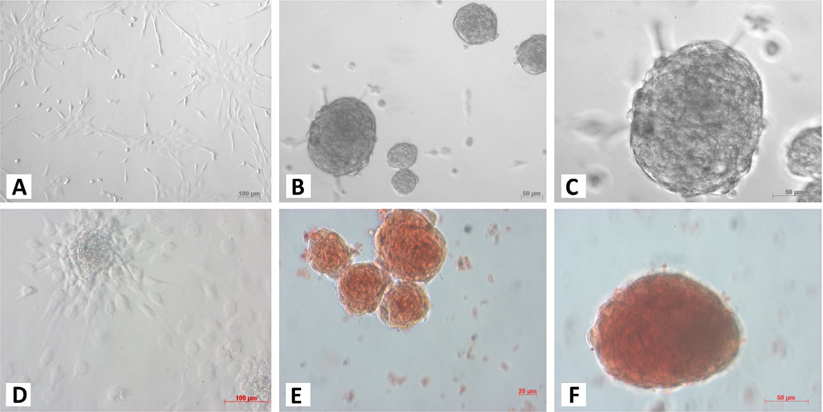
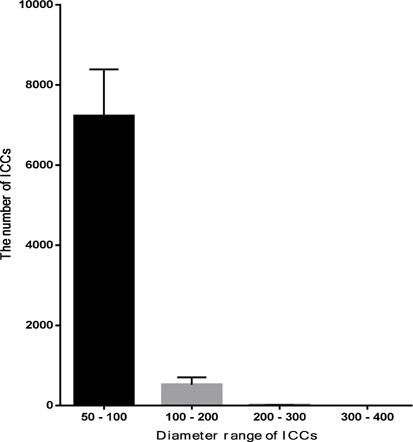
At the 1st stage of the differentiation process, ADSCs rapidly proliferated. These cells were confluent after 3–4 days of stimulation. At this time, fully adherent cells were trypsinized and re-seeded onto culture surfaces. Slow-dividing cells tended to aggregate as clusters at the end of the 2nd stage ( Figure 1A ). When differentiation was complete, the cells appeared similar to ICCs ( Figure 1B,C ). These clusters were positive for dithizone staining ( Figure 1E,F ). ICCs with diameters more than 50 μm were counted. A large number of ICCs had diameters of 50–100 μm. The number of ICCs decreased with increasing diameter. From 2 × 105 ADSCs cultured in a T-25 flask, at the end of procedure, there were 518 ± 108 ICCs at 100–200 μm, 9 ± 1 ICCs at 200–300 μm, and about 1 ± 0.5 ICCs at 300–400 μm diameter ( Figure 2 ). Interestingly, these suspension ICCs could be maintained for 60 days. When cultured in well-adherent surfaces, they could attach and expand slowly ( Figure 1D ).
Transcriptional expression of beta cell-related genes
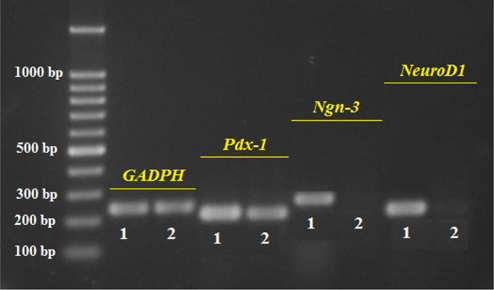
The results presented in Figure 3 show similar expression of the internal control (GAPDH) in ADSC and ICC samples, demonstrating successful RNA extraction and appropriate RT-PCR procedure. Pdx-1 expression in the ICCs is clearly higher than in the ADSCs while the expression of Ngn-3 and NeuroD1 is seen only in ICCs ( Figure 3 ). The positive expression of Pdx-1, Ngn-3, and Neuro-D1 prove that these islet cells are of beta cell lineage.
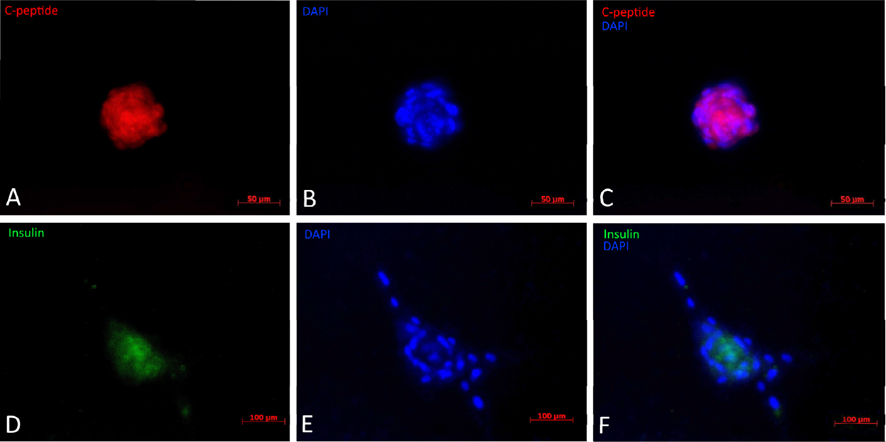
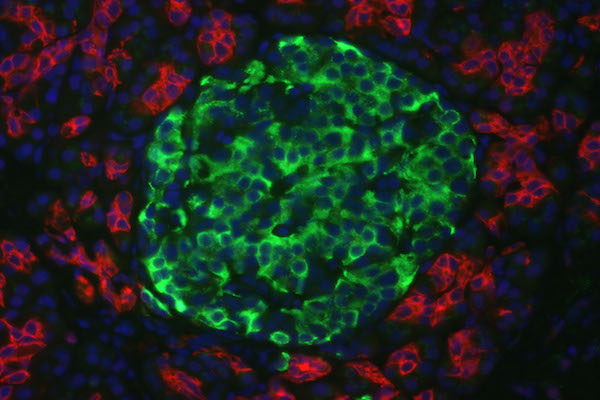
The results from immunofluorescent cytochemistry show that all obtained islets were positive for both insulin and C-peptide. As seen in Figure 4 ., islets were positive, with insulin staining visualized as green spots and C-peptide staining in red. The entire islet exhibited red color proving that they were able to synthesize proinsulin in their cytoplasm. Additionally, insulin expression is found in these differentiated clusters ( Figure 4 ). Therefore, they were characterized as beta cells.
Insulin secretion measurement
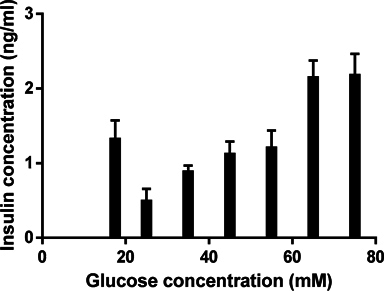
When the differentiation process was completed at the 3rd stage, the induced ICCs were evaluated functionally for insulin release capability. The results show that ICCs could produce and secrete insulin when induced in media supplemented with 17.5 mM glucose, and secreted insulin to approximately 1.327 ± 0.123 ng/ml. The results showed that insulin secretion gradually increased when glucose concentration was increased. In fact, at 25, 35, 45 and 55 mM, insulin concentration gradually increased from 0.497 ± 0.079 to 0.888 ± 0.040, 1.123 ± 0.084 and 1.208 ± 0.115 ng/ml, respectively. The insulin releasing level increased remarkably at glucose concentrations of 65 mM and 75 mM (2.151 ± 0.113 ng/ml at 65 mM and 2.180 ± 0.142 ng/ml at 75 mM) ( Figure 5 ).
Discussion
Stem cell therapy is one of the best candidates to treat degenerative diseases and disorders such as neural diseases and diabetes. The differentiation potential of stem cells has been studied to confirm their ability to replace dysfunctional cells and simultaneously supply a large number of functional cells for treatment. In order to recover pancreatic islet damage, insulinproducing cells have been effectively transplanted in diabetic cases with or without the use of stem cells Boroujeni, 2014 Chandra V 2011Gabr et al., 2013Zhang et al., 2011.
Insulin-producing cells can be differentiated from adult stem cells by gene transfection Qing-Song et al., 2012Thi-My Nguyen et al., 2014Wang et al., 2014 or induced soluble factors Gao et al., 2008Moshtagh et al., 2013Sun and Ji, 2009. Recently, adipose tissue derived stem cells have been the most favourable cell source for research and clinical application because of their dominant characteristics Bassi, 2012Choudhery et al., 2014Melief et al., 2013. Therefore, an important step forward for the treatment of diabetes would be to differentiate these cells into insulin-producing cells. In this study, we succesfully produced functional islets from ADSCs.
The three-step protocol of differentiation mimicked in vivo pancreatic formation and development. In the differentiation process, the structural changes of cells could be observed and divided into two phases, i.e., spheroidal cell cluster formation and functional maturation of the islet-like cells Okura et al., 2009Pokrywczynska et al.,2013. We found that the clusters appeared after 15 days of differentiation, a much longer period when compared to the differentiation of insulin-producing cells from embryonic stem cells Boyd et al., 2008. However, similar to our study, it took more than 10 days to induce the formation of clusters in the differentiation of insulin-producing cells from adipose tissue derived stromal cells by co-culture with isolated islets Karaoz et al., 2013. Zulewski, 2006 identified that mesenchymal stem cells trans-differentiated into insulin-producing cells via the expression of nestin, a neural stem cell marker. Therefore, in the first step and half of the second step of the differentiation protocol, it could be assumed that differentiation media containing extrinsic factors promoted the formation of neurosphere-like clusters. Supplementary N2 and B27 were used in order to enhance proliferation and maintain a low apoptosis rate of stem cells Dave et al., 2013. These factors could replace bovine serum without stress in the later step. In addition, they are components of neural differentiation media for embryonic and induced pluripotent stem cells. Therefore, it is possible that N2 and B27 play a role in trans-differentiation of mesenchymal cells into neurallike cells. Basic fibroblast growth factor (bFGF) has been shown to be useful in the differentiation of insulin-producing cells Wong, 2011. bFGF was shown to be secreted by endocrine precursor cells and to work as a chemoattractant to play a role in cell aggregation Hardikar et al., 2003. The other supplement, nicotinamide, has long been proven to be a potent inducer of endocrine differentiation Otonkoski et al., 1993 and used in most differentiation protocols to make insulin-producing cells. Many studies have confirmed that nicotinamide could promote islet cell proliferation Dave et al., 2013. In addition, insulin-transferrin-selenite (ITS) contributes to expand and increase the number of nestin-positive cells, which could differentiate into insulin expressing cells Okura et al., 2009Zulewski, 2006. We determined that media containing these factors could differentiate ADSCs into cells having a beta cell phenotype. After 30 days of differentiation, DTZ-positive clusters were observed, which were 50 – 400 μm in diameter and were similar in shape to human isolated islets Johnson et al., 2009.
These pseudo-islets were determined to express insulin and C-peptide in the cytoplasm by immunocytochemistry. Futhermore, they could respond to different glucose concentrations, among which 65 mM glucose stimulated a two-fold increase in release of insulin. Interestingly, it was also noted that the pseudo-islets grew larger with higher concentrations of glucose (data not shown). However, at concentrations of 75 mM or more, they dispersed as individual dead cells.
These results are consistent with the results of Dave and collaborators Dave et al.,2013. They assumed that the replication rate of beta cells increased with increasing glucose levels and at extremely hyperglycemic conditions, beta cell replication was reduced. We also demonstrated that our differentiated cluster cells expressed beta cell differentiation-related genes such as Pdx-1, Neuro-D1 and Ngn-3. Pancreatic and duodenal homeobox 1 (Pdx1) is involved in development of the pancreas, specifically for beta cell proliferation and survival Ben-Othman et al., 2013. Cells expressing Pdx-1 were confirmed to be definitive pancreatic precursors. Yuan and colleagues (2010) found that Pdx-1 played an effective role in differentiating mesenchymal stem cells into insulinproducing cells. The other transcriptional factor neurogenin-3 (Ngn-3), related to endocrine cell fate determination Ben-Othman et al., 2013, expressed clearly in differentiated cells. Moreover, the expression of downstream factors, like NeuroD1, was required for the differentiation of mono-hormone-producing cells Ben-Othman et al.,2013. These factors are also related to islet growth Pokrywczynska et al., 2013, beta cell maturation and glucose responsiveness Ben-Othman et al., 2013.
Finally, the established procotol can efficiently produce islets from ADSCs. We could produce about 500 islets from 2 × 105 ADSCs after 30 days. According to the islet transplantation procedure, about 4000 islet equivalents per kilogram of the recipient’s body weight were used to achieve clinical effects (Shapiro et al., 2000). That would imply that 50 × 106 ADSCs could produce enough islets for a diabetic mellitus patient with 50 kg body weight. This is a feasible solution in clinical application.
Conclusion
In conclusion, adipose tissue is an important soucre of stem cells for islet production. In this study, using a 3- step protocol, we produced approximately 500 islets from 2 × 105 ADSCs. These islets exhibited beta cell properties such as positive dithizone staining, expression of insulin and C-peptide in the cytoplasm, and expression of transcriptional factor genes related to beta cell differentiation (Pdx-1, Ngn3, Neuro-D). Importantly, the islet cells responded extremely well to glucose, in a concentration specific manner. These results provide confirmation of an efficient method for producing sufficient functional islets for clinical research and application.
Abbreviations
ADSCs: Adipose derived stem cells; FBS: Fetal bovine serum; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; ICCs: Islet-like cell clusters; IPCs: Insulin- producing cells; ITS: Insulin-transferrin-selenite; MSCs: Mesenchymal stem cells; NeuroD: Neurogenic differentiation 1; Ngn 3: Neurogenin 3; Pdx 1: Pancreatic duodenal homeobox 1; RT-PCR: Reverse transcriptase polymerase chain reaction; STZ: Dithizone dye; TMB: 3,3′,5,5′-Tetramethylbenzidine
References
-
Bassi.
Immune Regulatory Properties of Allogeneic Adipose- Derived Mesenchymal Stem Cells in the Treatment of Experimental Autoimmune Diabetes. Diabetes.
2012;
61
:
2534-2545
.
-
N.
Ben-Othman,
M.
Courtney,
A.
Vieira,
A.
Pfeifer,
N.
Druelle,
E.
Gjernes,
B.
Faurite,
F.
Avolio,
P.
Collombat.
From pancreatic islet formation to beta-cell regeneration.. Diabetes research and clinical practice 10.1016/j.diabres.2013.01.013.
2013
.
-
Boroujeni.
Human umbilical cord-derived mesenchymal stem cells can secrete insulin in vitro and in vivo. Biotechnol Appl Biochem.
2014;
61
:
82-92
.
-
A.S.
Boyd,
D.C.
Wu,
Y.
Higashi,
K.J.
Wood.
A comparison of protocols used to generate insulin-producing cell clusters from mouse embryonic stem cells. Stem cells.
2008;
26
:
1128-1137
.
-
V.
Chandra,
S.
G,
S.
Phadnis,
P.D.
Nair,
R.R.
Bhonde.
Generation of pancreatic hormone-expressing islet-like cell aggregates from murine adipose tissue-derived stem cells. Stem Cells.
2009;
27
:
1941-1953
.
-
S.G.
Chandra V,
Jaiswal AK
Muthyala S.
Islet-Like Cell Aggregates Generated from Human Adipose Tissue Derived Stem Cells Ameliorate Experimental Diabetes in Mice (doi:10.1371/journal.pone.0020615). PLoS ONE.
2011;
6(6)
:
e20615
.
-
M.S.
Choudhery,
M.
Badowski,
A.
Muise,
J.
Pierce,
D.T.
Harris.
Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. Journal of translational medicine.
2014;
12
:
8
.
-
S.D.
Dave,
A.V.
Vanikar,
H.L.
Trivedi.
Extrinsic factors promoting in vitro differentiation of insulin-secreting cells from human adipose tissue-derived mesenchymal stem cells. Appl Biochem Biotechnol.
2013;
170
:
962-971
.
-
S.D.
Dave,
A.V.
Vanikar,
H.L.
Trivedi.
In-vitro generation of human adipose tissue derived insulin secreting cells: up-regulation of Pax-6, Ipf-1 and Isl-1. Cytotechnology.
2014;
66
:
299-307
.
-
M.
Dominici,
K.
Le Blanc,
I.
Mueller,
I.
Slaper-Cortenbach,
F.
Marini,
D.
Krause,
R.
Deans,
A.
Keating,
D.
Prockop,
E.
Horwitz.
Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy.
2006;
8
:
315-317
.
-
I.A.
Fedyunina,
A.A.
Rzhaninova,
E.E.
Kirienko,
D.V.
Goldshtein.
Isolation of Insulin-Producing Cells from Different Populations of Multipotent Stromal Cells of the Umbilical Cord and Human Adipose Tissue. Kletochnye Tekhnologii v Biologii i.
2011;
Meditsine
:
No 1, 10-16
.
-
M.M.
Gabr,
M.M.
Zakaria,
A.F.
Refaie,
A.M.
Ismail,
M.A.
Abou-El- Mahasen,
S.A.
Ashamallah,
S.M.
Khater,
S.M.
El-Halawani,
R.Y.
Ibrahim,
G.S.
Uin.
Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocininduced diabetes in nude mice. Cell Transplant.
2013;
22
:
133-145
.
-
F.
Gao,
D.Q.
Wu,
Y.H.
Hu,
G.X.
Jin,
G.D.
Li,
T.W.
Sun,
F.J.
Li.
In vitro cultivation of islet-like cell clusters from human umbilical cord blood-derived mesenchymal stem cells. Translational research : the journal of laboratory and clinical medicine.
2008;
151
:
293-302
.
-
A.A.
Hardikar,
B.
Marcus-Samuels,
E.
Geras-Raaka,
B.M.
Raaka,
M.C.
Gershengorn.
Human pancreatic precursor cells secrete FGF2 to stimulate clustering into hormone-expressing islet-like cell aggregates. Proceedings of the National Academy of Sciences of the United States of America.
2003;
100
:
7117-7122
.
-
O.K.
Hwang,
E.Y.
Park,
L.D.
Thi Mai,
Y.M.
Choi,
H.S.
Jun.
Insulin-Producing Cells Differentiated from Human Adipose Tissue-Derived Stem Cells Ameliorate Hyperglycemia in Diabetic Mice. Tissue Engineering and Regenerative Medicine.
2011;
8
:
482-488
.
-
J.D.
Johnson,
Z.
Ao,
P.
Ao,
H.
Li,
L.-J.
Dai,
Z.
He,
M.
Tee,
K.J.
Potter,
A.M.
Klimek,
R.M.
Meloche.
Different Effects of FK506, Rapamycin, and Mycophenolate Mofetil on Glucose- Stimulated Insulin Release and Apoptosis in Human Islets. Cell transplantation.
2009;
18
:
833-845
.
-
E.
Karaoz,
A.
Okcu,
Z.S.
Unal,
C.
Subasi,
O.
Saglam,
G.
Duruksu.
Adipose tissue-derived mesenchymal stromal cells efficiently differentiate into insulin-producing cells in pancreatic islet microenvironment both in vitro and in vivo. Cytotherapy.
2013;
15
:
557-570
.
-
D.
Marappagounder,
I.
Somasundaram,
S.
Dorairaj,
R.J.
Sankaran.
Differentiation of mesenchymal stem cells derived from human bone marrow and subcutaneous adipose tissue into pancreatic islet-like clusters in vitro. Cell Mol Biol Lett.
2013;
18
:
75-88
.
-
S.M.
Melief,
J.J.
Zwaginga,
W.E.
Fibbe,
H.
Roelofs.
Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med.
2013;
2
:
455-463
.
-
M.L.
Mohamad Buang,
H.K.
Seng,
L.H.
Chung,
A.B.
Saim,
R.B.
Idrus.
In vitro generation of functional insulin-producing cells from lipoaspirated human adipose tissue-derived stem cells. Arch Med Res.
2012;
43
:
83-88
.
-
P.R.
Moshtagh,
S.H.
Emami,
A.M.
Sharifi.
Differentiation of human adipose-derived mesenchymal stem cell into insulin-producing cells: an in vitro study. J Physiol Biochem.
2013;
69
:
451-458
.
-
H.
Okura,
H.
Komoda,
Y.
Fumimoto,
C.M.
Lee,
T.
Nishida,
Y.
Sawa,
A.
Matsuyama.
Transdifferentiation of human adipose tissue-derived stromal cells into insulin-producing clusters. Journal of artificial organs : the official journal of the Japanese Society for Artificial Organs.
2009;
12
:
123-130
.
-
T.
Otonkoski,
G.M.
Beattie,
M.I.
Mally,
C.
Ricordi,
A.
Hayek.
Nicotinamide Is a Potent Inducer of Endocrine Differentiation. J Clin Invest.
1993;
92
:
1459-1466
.
-
M.
Pokrywczynska,
S.
Krzyzanowska,
A.
Jundzill,
J.
Adamowicz,
T.
Drewa.
Differentiation of stem cells into insulin-producing cells: current status and challenges. Archivum immunologiae et therapiae experimentalis.
2013;
61
:
149-158
.
-
G.
Qing-Song,
Z.
Ming-Yan,
W.
Lei,
F.
Xiang-Jun,
L.
Yu-Hua,
W.
Zhi- Wei,
Z.
Sha-Jun,
W.
Yao,
H.
Yan.
Combined Transfection of the Three Transcriptional Factors, PDX-1, NeuroD1, and MafA, Causes Differentiation of Bone Marrow Mesenchymal Stem Cells into Insulin-Producing Cells. Experimental Diabetes Research.
2012;
2012
:
10
.
-
N.
Sun,
H.
Ji.
In Vitro Differentiation of Human Placentaderived Adherent Cells into Insulin-producing Cells. Journal of International Medical Research.
2009;
37
:
400-406
.
-
P.
Thi-My Nguyen,
A.
Thai-Quynh Nguyen,
N.
Thi Nguyen,
N.
Thi-Minh Nguyen,
T.
Thi Duong,
N.
Hai Truong,
N.
Kim Phan.
Human umbilical cord blood derived mesenchymal stem cells were differentiated into pancreatic endocrine cell by Pdx-1 electrotransfer. Biomedical Research and Therapy.
2014;
1
:
1-7
.
-
K.
Timper,
D.
Seboek,
M.
Eberhardt,
P.
Linscheid,
M.
Christ-Crain,
U.
Keller,
B.
Müller,
H.
Zulewski.
Human adipose tissuederived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun.
2006;
24
:
1135-1140
.
-
P.
Van Pham,
N.B.
Vu,
N.L.-C.
Phan,
D.M.
Le,
N.C.
Truong,
N.H.
Truong,
K.H.-T.
Bui,
N.K.
Phan.
Good manufacturing practice-compliant isolation and culture of human adipose derived stem cells. Biomed Res Ther.
2014;
1
:
1-9
.
-
L.
Wang,
Y.
Huang,
Q.
Guo,
X.
Fan,
Y.
Lu,
S.
Zhu,
Y.
Wang,
X.
Bo,
X.
Chang,
M.
Zhu.
Differentiation of iPSCs into insulin-producing cells via adenoviral transfection of PDX-1, NeuroD1 and MafA. Diabetes Res Clin Pract.
2014;
104
:
383-392
.
-
R.S.
Wong.
Extrinsic factors involved in the differentiation of stem cells into insulin-producing cells: an overview. Experimental diabetes research.
2011;
2011
:
406182
.
-
H.
Yuan,
J.
Li,
N.
Xin,
Z.
Zhao,
G.
Qin.
Expression of Pdx1 mediates differentiation from mesenchymal stem cells into insulinproducing cells. Molecular biology reports.
2010;
37
:
4023-4031
.
-
S.
Zhang,
H.
Dai,
N.
Wan,
Y.
Moore,
Z.
Dai.
Promoting long-term survival of insulin-producing cell grafts that differentiate from adipose tissue-derived stem cells to cure type 1 diabetes. PLoS One.
2011;
6
:
e2970-6
.
-
H.
Zulewski.
Stem cells with potential to generate insulinproducing cells in man. Swiss med wkly.
2006;
136
:
647-654
.
-
L.
Dang,
A.
Bui,
V.
Pham,
N.
Phan,
P.
& Pham.
Production of islet-like insulin-producing cell clusters in vitro from adipose-derived stem cells. Biomedical Research And Therapy.
2015;
2(1)
:
184-192
.
Comments
Downloads
Article Details
Volume & Issue : Vol 2 No 1 (2015)
Page No.: 184-192
Published on: 2015-01-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 8062 times
- Download PDF downloaded - 1641 times
- View Article downloaded - 5 times
 Biomedpress
Biomedpress