Abstract
Objective: Tobacco smoking is one of the causes of the incidence and mortality of cancer in the world. This study aimed to review the relationship between TS and especially the use of cigarettes with common cancers of various organs of the body.
Methods: This study was conducted in English by November 2017 through a search in databases of the PubMed, Scopus and Web of Science. The search strategy included the key words "cancer", "tobacco smoke", "tobacco smoke", and "smoking." Articles that looked at the relationship between each type of cancer and smoking were entered into the study and summarized in Review.
Results: Tobacco smoking is associated with an increased risk of lung cancer, Upper aerodigestive tract, esophagus, stomach, bladder, kidneys, colorectal, prostate, and pancreas. However, further studies are needed to confirm the association between Tobacco smoking and liver, cervical, brain, gallbladder, Hodgkin’s disease, non-Hodgkin’s lymphoma and hematologic malignancies. However, Tobacco smoking plays a protective role in the development of thyroid cancers, skin and Kaposi’s sarcoma.
Conclusions: Given that almost all of the risk factors for most cancers are Tobacco smoking, increasing the public’s awareness of the harmful effects of smoking, implementing programs and policies to reduce smoking, can lead to a reduction in smoking and consequently reduce the resulting harmful consequences.
Introduction
Tobacco smoking (TS) is one of the causes of the incidence and mortality of cancer in the world [1]. It is responsible for about 25% of all cancers in men, 4% of all cancers in women and about 16% of all cancers in both sexes in most developed countries and 10% in less developed countries [2]. In the United States, approximately 40% of the cancers diagnosed is related to tobacco consumption [3]. According to recent research evidence, tobacco causes a lot of cases of lung cancer [4]. It is the leading cause of cancers of oral and throat, vocal cords, esophagus, stomach, kidneys, pancreas, liver, bladder, cervix, colon and rectum, and types of leukemia [2] [5] [6] [7] [8]. According to the Center for Cancer Prevention (CDC), tobacco-related cancers have been diagnosed between 2008 and 2013 for some 660000 people in the United States, but 343,000 of these people have died [9].
It seems that only small organs in the TS have been immune to cancer. Available evidence has shown that there is an inverse relationship between endometrium cancer and TS [2]. Evidence also suggests that the association between TS and liver cancer has not been definitively confirmed. Confounding variables such as hepatitis B and C can affect this relationship [10]. Given the numerous studies on smoking and cancer, this study aimed to review the relationship between TS and especially the use of cigarettes with common cancers of various organs of the body, taking into account possible confounding variables for each organ based on available evidence and review of the literature.
Methods
This study was conducted in English by November 2017 to include epidemiological evidence from all available randomized control trials, case-control and cohort studies reporting tobacco- related cancer risks through a search in databases of the PubMed, Scopus and Web of Science. The search strategy included the key words "cancer", "tobacco smoke", and "smoking." In addition, the reference lists of relevant articles were manually searched to find any other potentially eligible articles. Articles about smoking intensity are also included in the present study. We excluded reviews, commentaries, articles from overlapping samples, conference abstracts, and articles printed in languages other than English. Articles that looked at the relationship between each type of cancer and smoking were entered into the study and summarized in review. Most studies evaluated exposure to tobacco smoke quantitatively by reporting number of cigarettes per day, duration of smoking or age at starting to smoke, or in a cumulative way in terms of pack years 3 (number of packs of cigarettes smoked daily multiplied by the number of years of smoking). Similarly, the benefit of smoking cessation was evaluated either in comparing risks in former smokers with those in current smokers, or as a trend by years since quitting to smoke.
Results
Study characteristics
In the initial electronic literature search, 2540 articles were obtained from databases and 12 articles were obtained using manual search. After removing duplicates using Endnote X7 (n =1200), the title and abstract of the remaining 1340 articles were reviewed. After this stage, 191 articles were included in the study and 1164 of these articles were removed because of scientific reasons and lack of eligible criteria or unrelated to our aim, in all, 176 full papers were reviewed.
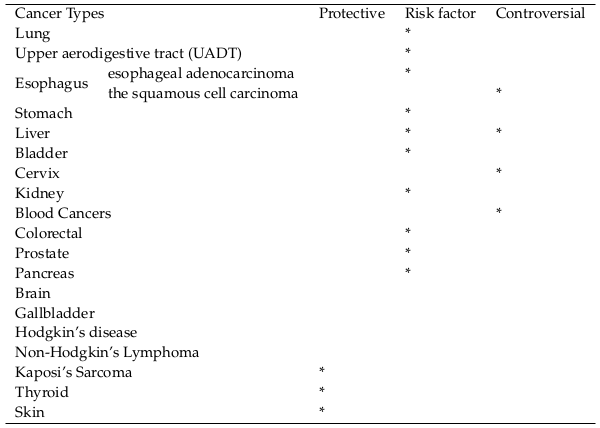
The most important cancers related to Tobacco Smoking are summarized in Table 1 .
Lung
One of the main causes of lung cancer (LC) is TS (4). Smokers are at risk to LC approximately 20 times more than people who have never smoked regularly. Smoking is responsible for 80% of LC in men and 50% of LC in women [11] . TS is associated with the incidence of small lung carcinoma (SCLC) and squamous cell carcinoma (SCC) [12]. Cigarette smoke contains about 20 potentially dangerous carcinogens and 3,500 chemical substances. The most common LC-induced chemicals found in cigarette smoke are polycyclic aromatic hydrocarbons (PAH) like benzo (a) pyrenes, and the tobacco-specific N-nitrosamine 4- (methylnitrosamino) -1- (3-pyridyl) -1-butanone NNK) [13]. Cigarette smoke also contains high levels of acrolin, which damages ciliated lining of the lung and helps to develop LC. Nitrogen oxides, acetaldehyde, phenols and formaldehyde are substances in cigarette smoke that indirectly contribute to LC [12]. Free radicals in cigarette smoke also cause oxidative damage in human and animal studies and contribute to the development of LC [14]. The risk of LC in the second hand smokers is also high [15] LC risk is 20-30% higher in non-smokers who marry smokers compared with others [11] [16] [17]. The risk of LC in heavy and continuous smokers is 20 to 50 times higher than non-smokers [18]. The most important risk index for LC is the duration of smoking [11].
Upper aerodigestive tract (UADT)
UADT cancers include oral, pharyngeal, hypopharynx, larynx, esophagus, nasal cavities, nasal sinuses, pharynx and adenocarcinoma of esophagus [2] [19]. The findings of a case-control study in 10 European countries showed in 2009 compared to non-smokers, TS is associated with a risk of UADT in general (OR: 6.72; 95% CI, 5.45-8.30), oral cavity cancer (OR: 5.83; 95% CI; 4.5-7.54), pharyngeal cancer (OR: 12.19; 95% CI, 8.29-17.92), and laryngeal and hypopharyngeal cancer (OR: 4017; 95% CI, 2.45-7.10 for esophagus) [19]. The findings of the meta-analysis study also showed that non-selective involuntary exposure to tobacco is associated with head and neck cancers, especially throat and larynx [20]. Based on a review study of nine case-control studies and a cohort study on sinonasal cancer, this type of cancer is associated with increased TS. Also, TS is also associated with a significant decrease in sinonasal cancer [2]. A finding from a study by Bosetti et al. (2007) showed that discontinuation of smoking at any age has reduced the risk of UADT incidence [21].
Esophagus
One of the major risk factors for esophageal cancer is TS [22]. The relationship between TS and esophageal cancer has been reported since 1985. It is also has been mainly related to squamous cell carcinoma of esophagus [2]. The findings of several case-control studies showed that TS is one of the proven risk factors for esophageal cancer [23] [24] [25]. Findings from several studies showed that the risk of developing ESCC in smokers is 3-7 times higher than non-smokers [26] [27]. According to a 2012 meta-analysis study, about 20-30% of esophageal cancer patients were addicted to cigarette smoking [28]. However, limited studies have directly evaluated the role of cigarette as a prognostic factor in esophageal cancer (22). Numerous studies in the United States, the incidence of esophageal adenocarcinoma in the United States and Britain in smokers has increased steadily compared with non-smokers. This process has been relatively constant for the squamous cell carcinoma and even has had a decreasing [29] [30].
Stomach
One of the known causes of stomach cancer is TS [31]. A systematic review study on 23 epidemiologic studies investigated the relationship between TS and stomach cancer with 10,290 cases and 26,145 controls revealed that compared to non-smokers, Odd Ratio(OR) for ever was
1.20 (95% CI: 1.09-1.32), OR for former smokers 1.12 (95% CI: 0.99-1.27) and OR for current smokers 1.25 (95% CI: 1.11-1.40). Also, with an increase in TS duration, the incidence of stomach cancer was significantly higher (OR, 95.1% CI 95% CI: 1.14-1.54) [32]. The findings of the cohort showed more than 215,000 men and women of the five ethnic groups (African Americans, Japanese Americans, Latino Americans, Native Hawaiians, and Whites), for all five racial groups, the rate of stomach cancer in smoker men (HR = 1.98; 95% CI 1.46-2.70) and in smoker women (HR = 1.78; 95% CI 1.23-2.57) was higher than non-smokers [33]. The findings of meta-analysis on cohort studies showed tobacco is one of the most important risk factors for stomach cancer [31]. The findings of a systematic review study on 10 cohort studies and 6 case-control studies revealed that there is a positive and strong association between TS and stomach cancer in men and there is a positive and weak correlation between TS and stomach cancer in women [34]. Since the middle of the last century, the incidence and mortality rate of stomach cancer in most of the high-income countries has declined [35][36]. Reducing the incidence of stomach cancer can be due to a reduction in the prevalence of smoking in men [31]. A positive association between TS and stomach cancer can be affected by confounding variables such as the infection of the stomach and the helicobacter pylori infection [37]. However, the association is not well known and confirmed [2].
Liver
Cohort and case-control studies have investigated the relationship between TS and liver cancer. Often, these studies have been conducted in areas where liver cancer is high, such as Southeast Asia and Europe [38,39]. Majority of these studies reported that the incidence of liver cancer among smokers was higher than non-smokers [40]. Other risk factors, such as hepatitis B or C and high alcohol intake, also play an important role in the development of liver cancer. Controlling these variables as a confounder also adjust the association between TS and liver cancer [39]. The International Agency for Research on Cancer also lists liver cancer in the list of tobacco- related malignancies [40]. A systematic review of 12 cohort studies showed that smoking is associated with an increase in the rate of liver cancer. However, there were no mention of potential confounders such as hepatitis B and C virus in these studies. In this study, 11 case-control studies were also studied and the findings showed that among the 11 case-control studies, 5 studies reported poor to moderate association between smoking and liver cancer, and the relation of dose response was only in one study. In the remaining 5 studies, the observed relationship was inverse or null due to the lack of data on the dose response variable [10]. Meta-analytical study findings in 2009 also supported the association between cigarette smoking and liver cancer [40].
Bladder
The risk of developing bladder cancer in smokers is 2 to 4 times more than non-smokers [41]. By increasing the amount and duration of smoking, the risk increases in both sexes [42–44]. Risk in people who use non-smoked cigarettes with high tar or black tobacco is more than those who use filtered cigarettes and golden tobacco with low tar [42]. Second-hand smokers have a 30-60% lower risk compared to smokers [18]. Smoking is responsible for 23% of BC in women and 50% of BC in men [45]. Following the discontinuation of smoking, the risk of bladder cancer decreases by 30-20% within 5 years. Recently, it has been argued that women may have more risk at bladder cancer due to TS [41]. The precise method of developing BC is not clear by the use of cigarettes. BC risk seems to be related to some of the chemicals found in cigarettes such as naphthylamine-2 and aminobiphenyl-4, polycyclic aromatic hydrocarbons [46]. Tobbaco combustion release at least 69 carcinogens including nitrosamine, polycyclicaromatic hydrocarbon, 2-naphthylamine and other aromatic amines, all of which are directly responsible for the role of mutagens in BC formation [47]. Also, the main routes of induction of cancer in smokers are the DNA adduct formation and genetic damage. They cause the change of specific cellular pathways, and subsequently uncontrolled cell growth and prevention of the activity of internal mechanisms inhibiting tumor growth [48].
Cervix
Smoking is one of the known risk factors for cervical cancer [49]. This role of smoking is independent of sexual behavior. some studies have shown a positive and significant relationship between the number of cigarettes consumed and the duration of smoking, and the incidence of cervical cancer. It seems that there is less danger in those who have left smoking [50][51]. However, other studies have not been able to relate their findings to smoking [52]-[54]. Meta-analysis findings in women with human papillomavirus revealed that smokers were twice as likely to have cervical cancer as non-smokers [55]. Cohort study findings in women with human papillomavirus also indicated that the risk of cervical cancer in women smokers and those who had previously smoked and now left smoking was at least three times more likely than other women [56]. The effect of direct TS carcinogens on cervix is based on the fact that nicotine metabolites can be 6 found in the cervical mucus of women who smoke [57]. Another acceptable mechanism is the suppression of local immune responses to human papillomavirus infection [58][59]. In general, further studies are needed to confirm the definitive effects of TS on the incidence of oral cancer.
Kidney
The findings of the epidemiological studies have shown the association between TS and the incidence of pelvis cancer. TS, especially cigarettes, has increased the risk of developing kidney cancers by 20%, which is seen more in middle-aged and elderly people. Cigarette smoking increases the risk of developing renal cell carcinoma (RCC). Meta-analysis study findings showed that the increased risk is associated with the number of cigarettes consumed [60]. The findings of another meta-analysis study showed that current smokers are at a 3 to 4-fold higher risk of developing urinary cancers than others. According to the study, in Europe about half of the cases of urinary tract cancer are detected in men and one third of women are attributed to TS [61]. With the cessation of smoking, the risk is reduced, but many years of time are needed to be as same as the risk level of people who quit smoking than the risk in people who have never smoked. The findings of several narrative studies have also recognized the significant role of smoking in the incidence of urinary tract cancer [62][63].
Blood Cancers
TS is not a risk factor for the risk of malignancies, but some epidemiological evidence supports the claim that TS is a risk factor for leukemia [64]. Some cohort and case studies have shown an increase in the incidence of hematologic malignancies, especially non- lymphoblastics, among smokers, while other studies have not shown significant correlation. However, the findings of Brownson et al (1993) [65], Siegel (1993) [66] and Sandler (1995) [67] showed that, despite the discrepancy in various studies, there is evidence that the relationship Between TS and the development of blood malignancies. TS contains about 3800 chemical substances, most of which are mutagenic and carcinogenic [68] and contain radioactive compounds and benzene, both of which are known carcinogens in the development of leukemia [2][68]]69]. It is estimated that smokers inhale benzene 10 times more than non-smokers [64]. Smoke also contains nitrosamines and urethane, which cause leukemia in animals, and also contain styrene and agricultural chemicals that are a potential source of leukemia in humans [68]. Available evidence also suggests that there is a relationship between TS and myeloid leukemia, and the risk increases with increasing the duration and amount of cigarette smoking [2].
Colorectal
Smoking, and in particular smoking, is a common risk factor for CRC in both genders [70]. Cohort studies in Korea showed that CRC risk was higher in non-smokers than in non-smokers [71]. Findings from the meta-analysis studies suggest a positive relationship between smoking, and colorectal adenoma and colorectal cancer. The findings from comprehensive systematic review of batteri et al. showed a significant relationship between smoking and the occurrence of adenoma polyps, which are pre-cancerous lesions of colorectal cancer [72]. The meta-analysis findings by Chen et al showed that smoking is a major risk factor for colorectal cancer [73]. Another study found that CRC risk increased in people who smoked over 45 years, while there was no increase in the number of cigarette packs with CRC risk [74]. moking is responsible for 20% of all types of CRC in the United States [75]. Several studies also found that smoking has increased the risk of CRC by up to 30% in smokers and women [76]–[78].
Prostate 7
Based on the findings of Paler et al., 2013, the incidence and death rate of PCa increased significantly with the increase in smoking [79]. Various mechanisms have been proposed for prostate cancer due to smoking [79][80]. Among the known mechanisms of tobacco use, it is possible to increase serum estrogen metabolites, and subsequently stimulation of an invasive tumor phenotype, which ultimately leads to prostate cancer [81].
Pancreas
One of the most important risk factors for pancreatic cancer is smoking [82][83]. Meta-analysis study findings revealed that the risk of pancreatic cancer in smokers is 2 times that of non- smokers. The risk of cancer is positively correlated with an increase in the number of cigarettes and duration of use [84]. About 20-30% of cases of pancreatic cancer are due to smoking. Most importantly, the risk of pancreatic cancer in people who leave smoking is reduced to near normal levels [85][86]. The findings of a meta-analysis study on 82 cohort studies and case-control cases published between 1950 and 2007 show that in current smokers, the relative risk (RR) of pancreatic septicemia is 1.7 (95% confidence interval [CI] 1.6-1.9) and in former smokers 1.2 (95% CI 1.1- 1.3) [84] . According to the international cohort study, the pancreatic cancer included 1481 cases and 1539 controls, RR in former smokers was 1. 1 (95% CI 0.9-1.3) and in current smokers was 1.8 (95% CI 1.4-2.3). This risk was also associated with an increase in the number of cigarettes and the duration of use. As in individuals who smoked 30 or more doses daily, RR was 1.75 and in those who smoked at least 50 years, while in people who had left smoking for more than 15 years, RR was similar to those who never smoked [87].
Brain
Two types of common brain tumors are glioma and meningioma, accounting for approximately 75% of all brain tumors [88]. There is contradictory evidence regarding the relationship between smoking and gliomas or meningitis. In smokers, tobacco smoke is the largest source of N-nitroso compounds [89][90]. These compounds are associated with the development of tumors in the nervous system in animals [90][91]. Despite the role of nicotine in increasing the blood brain barrier permeability [92], whether N-nitrose penetrates into the brain or not is still unknown [93]. Considering that TS causes cancer in various organs of the body, tobacco smoke is thought to be effective in treating brain tumors [88]. However, the findings of most case-control studies have not shown evidence of increased glioma risk associated with TS [94]–[96]. Some other studies have shown RR increases, but these findings are not meaningful or limited to specific population subgroups [97]–[99]. In cohort studies, some findings also did not report a relationship between TS and brain tumors [100]–[102], while others reported a meaningful relationship only in women [103][104]. Meta-analysis study findings have recently shown that there is no relationship between cigarette smoke and glioma in general, though there was a slight, but significant increase in one cohort study entered into the analysis. For meningioma, some case-control studies did not report evidence of increased risk [96][102][105]–[108], and other studies reported association only in one sex [95][109][110]. In sum, there is no clear evidence of the link between tobacco smoke and glioma and meningitis, and further studies are needed.
Gallbladder
Researchers believe that TS affects hepatobiliary system and is associated with increased risk of gallbladder cancer [111][112]. However, studies on the association between gallbladder disease and gallstone risk are as a mix. Several case-study and cross-sectional descriptive studies did not confirm the association [113]–[115] or even inverse relationship [116], while quantitative studies showed increased risk [117][118]. Some findings from these studies seem to be influenced by the selection or recall biased. Therefore, the final conclusion based on such studies is difficult and further studies are needed with more robust design, such as cohort studies.
Hodgkin’s disease
TS is one of the potential risk factors for the classic Hodgkin’s lymphoma (CHL), which requires careful examination. Despite the limited number of studies in this regard, the findings of these studies are inconsistent. The findings of some studies have shown a significant decrease [119], some lack of association [120][123], and some significant increases [124]–[126] in overall HL risk in smokers. On the other hand, the discrepancy in the findings may be due to various methodological issues, including the low sample size of the study, the biases associated with the selection of the group, and the participants in the study and the uncontrollable confounders. Recently, the findings of three meta-analysis studies showed that TS increased the overall risk of HL [127]–[129]. Overall, it can be concluded that TS is one of the moderating risk factors for CHL types.
Non-Hodgkin’s Lymphoma
The findings of the early epidemiological studies on the role of smoking in non-Hodgkin’s lymphoma have been disconnected. Some studies reported that smoking was associated with the incidence of this cancer [130]–[134], while others found that there was no relationship between cigarette smoke and non-Hodgkin’s lymphoma [135]–[140]. Meta-analysis study findings in 2005 indicated that smoking is associated with a slight increase in non-Hodgkin’s lymphoma (OR, 1.07; 95% CI, 1.00-1.15) [141]. Although smoking is generally unrelated to the risk of non-Hodgkin’s lymphoma, there is much evidence that smoking can be associated with the follicular lymphoma subtype, especially in women [126][142]–[145]. More recently, cohort study findings showed that people who were exposed to tobacco smoke 40 years or more became 50%more likely to develop non-Hodgkin’s lymphoma than those who smoked 5 years or less [146]. There are more studies with more robust design like cohort studies in this area.
Kaposi’s Sarcoma
Kaposi’s sarcoma is a relatively rare type of skin cancer that is common in humans. It is not activated in the normal state of the body, it can be considered an opportunistic disease [147]. The common feature of these diseases is its suppression by the immune system in the normal state of the body and its activation in the event of immunodeficiency. Unlike other cancers that smoke is a risk factor for them, it is known as a protective agent in this cancer [147][148]. However, many studies have not been done in this area.
The findings of the case-control study showed that there is a correlation between current cigarette smoking and a reduction in the incidence of Kaposi’s sarcoma (OR, 0.20; 95% CI, 0.06-0.67) [149]. This inverse relationship is supported by reducing the risk of lung cancer in these patients [150]–[153]. In a cohort study, Nawar etal reported a lower relative risk (OR, 0.6, 95% CI, 0.5-0.9) in the incidence of Acquired Immune Deficiency Syndrome (AIDS) and Kaposi’s syndrome in smokers [147]. Immunological effects of smoking are thought to reduce the risk of Kaposi’s sarcoma. For example, nicotine can affect dendrite cellular function [154][155], and may cause cytokine changes and produce growth factor involved in Kaposi’s sarcoma development [156]. However, according to a clinical trial that used nicotine skin patches for 15 weeks to improve skin lesions of Kaposi’s sarcoma, it showed a small effect of nicotine [155]. Also, the findings of Guttman et al. And Conley et al. did not confirm the inverse relationship between smoking and Kaposi’s sarcoma [157][158]. In general, given that most patients with Kaposi’s sarcoma are often at an advanced age, their use of cigarettes increases the incidence of smoking- related illnesses and increases the mortality of these patients and thus cannot be considered as a completely protective factor to be considered [149]. On the other hand, in order to confirm the protective effect of smoking, the need for more comprehensive cohort studies is felt.
Skin
Skin cancer is one of the most common and prevalent types of cancers in humans. Three major types of skin malignancies include basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and melanoma [148]. TS contains several carcinogenic compounds that affect at least 18 types of cancers [159] but have a reversible relationship with skin malignancies. Smoking can reduce the blood flow of the skin and stop the immune response, which increases the risk of skin cancer [160]; on the other hand, it can protect the inflammatory response induced by UV radiation and, therefore, reduce it. Risk of skin cancer [161]–[165]. his effect of smoking is relatively due to the prolonged effects of nicotine accumulation in human tissues that contain melanin [166]. Various studies have shown that transdermel delivery of nicotine suppresses inflammatory responses to sodium lauryl sulphate and UV-B [167][168]. Cohort study findings showed that in male ever smokers had less common risk of developing melanoma (RR = 0.72; 95% CI: 0.58-0.86) and BCC (RR = 0.94; 95) compared to those who never smoked. However, there was no significant relationship with SCC (RR = 0.99; 95% CI: 1.03-1.08) [148]. Findings from Mills Case Studies [168] and Kessides [164] also suggest that cigarette smoking has an inverse association with melanoma, although this relationship was not significant. Another study found that there was an insignificant decrease in the incidence of melanoma in men who were ever smokers (RR = 0.6; 95% CI: 0.3-1.1), but not seen in women (RR = 1.0; 95% CI: 0.3-1.1. [169].
Thyroid
There is some evidence that smoking is a significant modifiable treatment that affects thyroid cancer. The findings of several case-control [170]–[173] and prospective [173][174] studies have shown that there is an inverse association between thyroid cancer and smoking, but some studies did not confirm this relationship [175][177]. The findings of a meta-analysis study on five cohort studies indicate that there is an inverse association between current cigarette smoking and the risk of thyroid cancer. Based on this study, if the severity and duration of smoking is low in former smokers [178], the risk will be less. The findings of another meta-analysis study showed that the risk of thyroid cancer in current smokers decreased by 40% [179]. Compared to people who have never smoked or smoked, current smokers have lower levels of thyroid stimulating hormone (TSH) [180][181], Triiodothyronine (T3) [181] and thyroxine (T4) [180] and a lower prevalence of serum autistic thyroiditis [180]. T3, T4 and especially TSH play a major role in thyroid cancer [178][182]. Smoking is also effective in changing the levels of sex steroid hormones on thyroid cancer [183][184].
Conclusion
The purpose of this review study was to investigate the relationship between TS and especially smoking and common cancers. Based on the results of studies, TS is associated with an increased risk of lung cancer, Upper aerodigestive tract, esophagus, stomach, bladder, kidneys, colorectal, prostate, and pancreas. However, further studies are needed to confirm the association between TS and liver, cervical, brain, gallbladder, Hodgkin’s disease, non-Hodgkin’s lymphoma and hematologic malignancies. However, TS plays a protective role in the development of thyroid cancers, skin and Kaposi’s sarcoma. Given that almost all of the risk factors for most cancers are TS, increasing the public’s awareness of the harmful effects of smoking, implementing programs and policies to reduce smoking, can lead to a reduction in smoking and consequently reduce the resulting harmful consequences. There are also some limitations in this study. This review is not a systematic review and only English articles were reviewed.
List of abbreviations
AIDS: Acquired Immune Deficiency Syndrome; BCC: Basal cell carcinoma; CDC: Center for Cancer Prevention; CHL: Classic Hodgkin’s lymphoma; CRC: Colorectal Cancer; LC: Lung cancer; PCa: Prostate cancer; RR: Relative risk; RCC: Renal cell carcinoma; SCLC: Small lung carcinoma; SCC: Squamous cell carcinoma; T4: Thyroxine; TS: Tobacco smoking; T3: Triiodothyronine; UADT: Upper aerodigestive tract.
Authors’ contributions
All authors contributed to the design of the research, Y.K, N.P.A, M.A, O.O, M.SE, extracted the data and summarized it. All authors drafted the first version. HSG, M.V and HS edited the first draft. All authors reviewed, commented and approved the final draft.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Competing interests
The authors declare that no competing interests exist.
References
-
Prevention
for Disease CC.
Annual smoking-attributable mortality, years of potential life lost, and economic costs-united States, 1995-1999. MMWR. Morbidity and Mortality Weekly Report.
2002;
51
:
300-3
.
PubMed Google Scholar -
AJ
Sasco,
MB
Secretan,
K
Straif.
Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer.
2004;
(Amsterdam
:
Netherlands) 45
.
View Article PubMed Google Scholar -
N
Statistics.
National vital statistics report. 2016
.
-
CA
Pope,
RT
Burnett,
MC
Turner,
A
Cohen,
D
Krewski,
M
Jerrett.
Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environmental Health Perspectives.
2011;
119
:
1616-21
.
View Article PubMed PMC Google Scholar -
D
Yadav,
AB
Lowenfels.
The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology.
2013;
144
:
1252-61
.
View Article PubMed PMC Google Scholar -
SC
Chuang,
YC
Lee,
M
Hashibe,
M
Dai,
T
Zheng,
P
Boffetta.
Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: a meta-analysis. Cancer.
2010;
Epidemiology
:
Biomarkers & Prevention 19
.
View Article PubMed PMC Google Scholar -
D
Limsui,
RA
Vierkant,
LS
Tillmans,
AH
Wang,
DJ
Weisenberger,
PW
Laird.
Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. Journal of the National Cancer Institute.
2010;
102
:
1012-22
.
View Article PubMed PMC Google Scholar -
HS
Gandomani,
AA
Tarazoj,
H
Salehiniya.
Cigarette: The Silent Killer in the World. Biomedical Research and Therapy.
2017;
4
:
1624-8
.
View Article Google Scholar -
Centers for Disease Control Prevention.
Classification of Diseases, Functioning, and Disability. https://wwwcdcgov/media/releases/2016/p1110-vital-signs-cancer-tobacco.html.
2016;
206
.
-
K
Tanaka,
I
Tsuji,
K
Wakai,
C
Nagata,
T
Mizoue,
M
Inoue,
DRG
for the,
JE
of Cancer Prevention Strategies in.
Cigarette smoking and liver cancer risk: an evaluation based on a systematic review of epidemiologic evidence among Japanese. Japanese Journal of Clinical Oncology.
2006;
36
:
445-56
.
View Article PubMed Google Scholar -
H
Sadeghi-Gandomani,
A
Asgari-Tarazoj,
M
Ghoncheh,
SM
Yousefi,
M
Delaram,
H
Salehiniya.
Lung cancer in the world: the incidence, mortality rate and risk factors. World cancer research journal.
2017;
4
.
-
M
Furrukh.
Tobacco smoking and lung cancer: perception-changing facts. Sultan Qaboos University Medical Journal.
2013;
13
:
345-58
.
View Article PubMed PMC Google Scholar -
S
Couraud,
G
Zalcman,
B
Milleron,
F
Morin,
PJ
Souquet.
Lung cancer in never smokers-a review. European Journal of Cancer.
2012;
(Oxford
:
England) 48
.
View Article PubMed Google Scholar -
S
Kligerman,
C
White.
Epidemiology of lung cancer in women: risk factors, survival, and screening. AJR. American Journal of Roentgenology.
2011;
196
:
287-95
.
-
P
Vineis,
G
Hoek,
M
Krzyzanowski,
F
Vigna-Taglianti,
F
Veglia,
L
Airoldi.
Lung cancers attributable to environmental tobacco smoke and air pollution in non-smokers in different European countries: a prospective study. Environmental Health.
2007;
6
:
7
.
View Article Google Scholar -
AK
Hackshaw,
MR
Law,
NJ
Wald.
The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ (Clinical Research Ed.).
1997;
315
:
980-8
.
-
P
Boffetta.
Involuntary smoking and lung cancer. Scandinavian Journal of Work, Environment & Health.
2002;
28
:
30-40
.
PubMed Google Scholar -
J
Cornfield,
W
Haenszel,
EC
Hammond,
AM
Lilienfeld,
MB
Shimkin,
EL
Wynder.
Smoking and lung cancer: recent evidence and a discussion of some questions. 1959. International Journal of Epidemiology.
2009;
38
:
1175-91
.
-
YC
Lee,
M
Marron,
S
Benhamou,
C
Bouchardy,
W
Ahrens,
H
Pohlabeln.
Active and involuntary tobacco smoking and upper aerodigestive tract cancer risks in a multicenter case- control study. Cancer.
2009;
Epidemiology
:
Biomarkers & Prevention 18
.
View Article PubMed PMC Google Scholar -
A
Wyss,
M
Hashibe,
SC
Chuang,
YC
Lee,
ZF
Zhang,
GP
Yu.
Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. American Journal of Epidemiology.
2013;
178
:
679-90
.
View Article PubMed PMC Google Scholar -
C
Bosetti,
S
Gallus,
R
Peto,
E
Negri,
R
Talamini,
A
Tavani.
Tobacco smoking, smoking cessation, and cumulative risk of upper aerodigestive tract cancers. American Journal of Epidemiology.
2008;
167
:
468-73
.
View Article PubMed Google Scholar -
J
Kuang,
Z
min Jiang,
Y
xian Chen,
W
peng Ye,
Q
Yang,
H
zhong Wang,
D
rong Xie.
Smoking exposure and survival of patients with esophagus cancer: a systematic review and meta-analysis. Gastroenterology research and practice 2016.
2016
.
-
I
Tramacere,
CL
Vecchia,
E
Negri.
Tobacco smoking and esophageal and gastric cardia adenocarcinoma: a meta-analysis. Epidemiology (Cambridge, Mass.).
2011;
22
:
344-9
.
-
Y
Lin,
Y
Totsuka,
Y
He,
S
Kikuchi,
Y
Qiao,
J
Ueda.
Epidemiology of esophageal cancer in Japan and China. Journal of Epidemiology.
2013;
23
:
233-42
.
View Article PubMed PMC Google Scholar -
J
Steevens,
LJ
Schouten,
RA
Goldbohm,
PA
van den Brandt.
Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: a prospective cohort study. Gut.
2010;
59
:
39-48
.
View Article Google Scholar -
M
Morita,
R
Kumashiro,
N
Kubo,
Y
Nakashima,
R
Yoshida,
K
Yoshinaga.
Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: epidemiology, clinical findings, and prevention. International Journal of Clinical Oncology.
2010;
15
:
126-34
.
View Article PubMed Google Scholar -
F
Islami,
V
Fedirko,
I
Tramacere,
V
Bagnardi,
M
Jenab,
L
Scotti.
Alcohol drinking and esophageal squamous cell carcinoma with focus on light-drinkers and never-smokers: a systematic review and meta-analysis. International Journal of Cancer.
2011;
129
:
2473-84
.
View Article PubMed Google Scholar -
I
Ferronha,
A
Bastos,
N
Lunet.
Prediagnosis lifestyle exposures and survival of patients with gastric cancer: systematic review and meta-analysis. European Journal of Cancer Prevention.
2012;
21
:
449-52
.
View Article PubMed Google Scholar -
MB
Cook,
F
Kamangar,
DC
Whiteman,
ND
Freedman,
MD
Gammon,
L
Bernstein.
Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. Journal of the National Cancer Institute.
2010;
102
:
1344-53
.
View Article PubMed PMC Google Scholar -
ZF
Zhang,
RC
Kurtz,
JR
Marshall.
garette smoking and esophageal and gastric cardia. 1997
.
-
R
Ladeiras-Lopes,
AK
Pereira,
A
Nogueira,
T
Pinheiro-Torres,
I
Pinto,
R
Santos-Pereira.
Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes & Control.
2008;
19
:
689-701
.
View Article PubMed Google Scholar -
C
Pelucchi,
N
Lunet,
S
Boccia,
ZF
Zhang,
D
Praud,
P
Boffetta.
The stomach cancer pooling (StoP) project: study design and presentation. European Journal of Cancer Prevention.
2015;
24
:
16-23
.
View Article Google Scholar -
AM
Nomura,
LR
Wilkens,
BE
Henderson,
M
Epplein,
LN
Kolonel.
The association of cigarette smoking with gastric cancer: the multiethnic cohort study. Cancer Causes & Control.
2012;
23
:
51-8
.
View Article PubMed PMC Google Scholar -
Y
Nishino,
M
Inoue,
I
Tsuji,
K
Wakai,
C
Nagata,
T
Mizoue,
DRG
for the,
JE
of Cancer Prevention Strategies in.
Tobacco smoking and gastric cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Japanese Journal of Clinical Oncology.
2006;
36
:
800-7
.
View Article PubMed Google Scholar -
M
Malvezzi,
M
Bonifazi,
P
Bertuccio,
F
Levi,
CL
Vecchia,
A
Decarli.
An age-period-cohort analysis of gastric cancer mortality from 1950 to 2007 in Europe. Annals of Epidemiology.
2010;
20
:
898-905
.
View Article PubMed Google Scholar -
D
Schottenfeld,
JF
Fraumeni.
Cancer epidemiology and prevention. 2006
.
-
C
IAfRo.
IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. In v. 42: Silica and some silicates.
1987
.
-
FX
Bosch,
J
Ribes,
M
Diaz,
R
Cleries.
Primary liver cancer: worldwide incidence and trends. Gastroenterology.
2004;
127
:
S5-16
.
View Article PubMed Google Scholar -
SC
Chuang,
CL
Vecchia,
P
Boffetta.
Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Letters.
2009;
286
:
9-14
.
View Article PubMed Google Scholar -
YC
Lee,
C
Cohet,
YC
Yang,
L
Stayner,
M
Hashibe,
K
Straif.
Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. International Journal of Epidemiology.
2009;
38
:
1497-511
.
View Article PubMed Google Scholar -
MP
Zeegers,
FE
Tan,
E
Dorant,
PA
van Den Brandt.
The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer.
2000;
89
:
630-9
.
View Article Google Scholar -
ND
Freedman,
DT
Silverman,
AR
Hollenbeck,
A
Schatzkin,
CC
Abnet.
Association between smoking and risk of bladder cancer among men and women. Journal of the American Medical Association.
2011;
306
:
737-45
.
View Article PubMed PMC Google Scholar -
DO
Escudero,
SP
Shirodkar,
VB
Lokeshwar.
Bladder carcinogenesis and molecular pathways. Bladder Tumors.
2011
.
-
Institute
The Scripps Research.
Bladder Cancer, [Internet] [cited 29 june]. 2017
.
-
MP
Zeegers,
E
Kellen,
F
Buntinx,
PA
van den Brandt.
The association between smoking, beverage consumption, diet and bladder cancer: a systematic literature review. World Journal of Urology.
2004;
21
:
392-401
.
View Article PubMed Google Scholar -
HS
Gandomani,
AA
Tarazoj,
FH
Siri,
AK
Rozveh,
S
Hosseini,
NN
Borujeni.
Essentials of bladder cancer worldwide: incidence, mortality rate and risk factors. Biomedical Research and Therapy.
2017;
4
:
1638-55
.
View Article Google Scholar -
null
null.
2012 Bladder cancer risk from occupational and environmental exposures. Urologic Oncology: Seminars and Original Investigations.
2012
.
-
AJ
Alberg,
JR
Hebert.
Cigarette smoking and bladder cancer: a new twist in an old saga?. 2009
.
-
HC
Vet,
F
Sturmans,
PG
Knipschild.
The role of cigarette smoking in the etiology of cervical dysplasia. Epidemiology (Cambridge, Mass.).
1994;
5
:
631-3
.
-
J
Brisson,
C
Morin,
M
Fortier,
M
Roy,
C
Bouchard,
J
Leclerc.
Risk factors for cervical intraepithelial neoplasia: differences between low- and high-grade lesions. American Journal of Epidemiology.
1994;
140
:
700-10
.
View Article PubMed Google Scholar -
L
Kjellberg,
G
Hallmans,
AM
Ahren,
R
Johansson,
F
Bergman,
G
Wadell.
Smoking, diet, pregnancy and oral contraceptive use as risk factors for cervical intra-epithelial neoplasia in relation to human papillomavirus infection. British Journal of Cancer.
2000;
82
:
1332-8
.
View Article PubMed PMC Google Scholar -
LA
Koutsky,
KK
Holmes,
CW
Critchlow,
CE
Stevens,
J
Paavonen,
AM
Beckmann.
A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. The New England Journal of Medicine.
1992;
327
:
1272-8
.
View Article PubMed Google Scholar -
AO
Olsen,
K
Gj?en,
T
Sauer,
I
rstavik,
O
Naess,
K
Kierulf.
Human papillomavirus and cervical intraepithelial neoplasia grade II-III: a population-based case-control study. International Journal of Cancer.
1995;
61
:
312-5
.
View Article PubMed Google Scholar -
M
Schiff,
TM
Becker,
M
Masuk,
L
van Asselt-King,
CM
Wheeler,
KK
Altobelli.
Risk factors for cervical intraepithelial neoplasia in southwestern American Indian women. American Journal of Epidemiology.
2000;
152
:
716-26
.
View Article PubMed Google Scholar -
X
Castellsague,
FX
Bosch,
N
Munoz.
Environmental co-factors in HPV carcinogenesis. Virus Research.
2002;
89
:
191-9
.
View Article Google Scholar -
PE
Castle,
S
Wacholder,
AT
Lorincz,
DR
Scott,
ME
Sherman,
AG
Glass.
A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. Journal of the National Cancer Institute.
2002;
94
:
1406-14
.
View Article PubMed Google Scholar -
MH
Schiffman,
NJ
Haley,
JS
Felton,
AW
Andrews,
RA
Kaslow,
WD
Lancaster.
Biochemical epidemiology of cervical neoplasia: measuring cigarette smoke constituents in the cervix. Cancer Research.
1987;
47
:
3886-8
.
PubMed Google Scholar -
JM
Palefsky,
EA
Holly.
Molecular virology and epidemiology of human papillomavirus. 1995
.
-
JT
Cox.
Epidemiology of cervical intraepithelial neoplasia: the role of human papillomavirus. Baillieres´ Clinical Obstetrics and Gynaecology.
1995;
9
:
1-37
.
View Article Google Scholar -
M
Zeegers,
FE
Tan,
E
Dorant,
PA
van den Brandt.
The impact of characteristics of cigarette smoking on urinary tract cancer risk. Cancer.
2000;
89
:
630-639
.
View Article Google Scholar -
PH
Chyou,
AM
Nomura,
GN
Stemmermann.
A prospective study of diet, smoking, and lower urinary tract cancer. Annals of Epidemiology.
1993;
3
:
211-6
.
View Article Google Scholar -
J
SL,
C
SM.
Epidemiology and etiology of bladder cancer. In Seminars in surgical oncology.
1997
.
-
T
Shirai,
Y
Fradet,
H
Huland,
C
Bollack,
M
Droller,
R
Janknegt.
The etiology of bladder cancer-are there any new clues or predictors of behavior?. International Journal of Urology.
1995;
2
:
64-75
.
View Article PubMed Google Scholar -
P
Pasqualetti,
V
Festuccia,
P
Acitelli,
A
Collacciani,
A
Giusti,
R
Casale.
Tobacco smoking and risk of haematological malignancies in adults: a case-control study. British Journal of Haematology.
1997;
97
:
659-62
.
View Article PubMed Google Scholar -
RC
Brownson,
TE
Novotny,
MC
Perry.
Cigarette smoking and adult leukemia. A meta- analysis. Archives of Internal Medicine.
1993;
153
:
469-75
.
-
M
Siegel.
Smoking and leukemia: evaluation of a causal hypothesis. American Journal of Epidemiology.
1993;
138
:
1-9
.
View Article PubMed Google Scholar -
DP
Sandler.
Recent studies in leukemia epidemiology. Current Opinion in Oncology.
1995;
7
:
12-8
.
View Article PubMed Google Scholar -
C
Magnani,
G
Pastore,
L
Luzzatto,
B
Terracini.
Parental occupation and other environmental factors in the etiology of leukemias and non-Hodgkins´ lymphomas in childhood: a case-control study. Tumori.
1990;
76
:
413-9
.
View Article PubMed Google Scholar -
P
Vineis,
N
Caporaso.
Tobacco and cancer: epidemiology and the laboratory. Environmental Health Perspectives.
1995;
103
:
156-60
.
View Article PubMed PMC Google Scholar -
P
Pasqualetti,
R
Casale,
D
Colantonio,
A
Collacciani.
Occupational risk for hematological malignancies. American Journal of Hematology.
1991;
38
:
147-9
.
View Article PubMed Google Scholar -
HS
Gandomani,
M
Aghajani,
A
Mohammadian-Hafshejani,
AA
Tarazoj,
V
Pouyesh,
H
Salehiniya.
Colorectal cancer in the world: incidence, mortality and risk factors. Biomedical Research and Therapy.
2017;
4
:
1656-75
.
View Article Google Scholar -
HJ
Kim,
SM
Lee,
NK
Choi,
SH
Kim,
HJ
Song,
YK
Cho,
BJ
Park.
Smoking and colorectal cancer risk in the Korean elderly. Journal of preventive medicine and public health= Yebang Uihakhoe chi.
2006;
39
:
123-129
.
-
E
Botteri,
S
Iodice,
S
Raimondi,
P
Maisonneuve,
AB
Lowenfels.
Cigarette smoking and adenomatous polyps: a meta-analysis. Gastroenterology.
2008;
134
:
388-395e3
.
-
K
Chen,
JL
Qiu,
Y
Zhang,
YW
Zhao.
Meta analysis of risk factors for colorectal cancer. World Journal of Gastroenterology.
2003;
9
:
1598-600
.
View Article PubMed PMC Google Scholar -
S
Cho,
A
Shin,
SK
Park,
HR
Shin,
SH
Chang,
KY
Yoo.
Alcohol drinking, cigarette smoking and risk of colorectal cancer in the Korean multi-center cancer cohort. Journal of Cancer Prevention.
2015;
20
:
147-52
.
View Article PubMed PMC Google Scholar -
CS
Fuchs,
GA
Colditz,
MJ
Stampfer,
EL
Giovannucci,
DJ
Hunter,
EB
Rimm.
A prospective study of cigarette smoking and the risk of pancreatic cancer. Archives of Internal Medicine.
1996;
156
:
2255-60
.
View Article PubMed Google Scholar -
E
Giovannucci,
EB
Rimm,
MJ
Stampfer,
GA
Colditz,
A
Ascherio,
J
Kearney.
A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. Journal of the National Cancer Institute.
1994;
86
:
183-91
.
-
PA
Newcomb,
BE
Storer,
PM
Marcus.
Cigarette smoking in relation to risk of large bowel cancer in women. Cancer Research.
1995;
55
:
4906-9
.
PubMed Google Scholar -
ED
Paskett,
KW
Reeves,
TE
Rohan,
MA
Allison,
CD
Williams,
CR
Messina.
Association between cigarette smoking and colorectal cancer in the Womens´ Health Initiative. Journal of the National Cancer Institute.
2007;
99
:
1729-35
.
View Article PubMed Google Scholar -
CJ
Paller,
X
Ye,
PJ
Wozniak,
BK
Gillespie,
PR
Sieber,
RH
Greengold.
A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer and Prostatic Diseases.
2013;
16
:
50-5
.
View Article PubMed PMC Google Scholar -
J
Chen,
Y
Song,
L
Zhang.
Lycopene/tomato consumption and the risk of prostate cancer: a systematic review and meta-analysis of prospective studies. Journal of Nutritional Science and Vitaminology.
2013;
59
:
213-23
.
View Article Google Scholar -
E
Giovannucci,
EB
Rimm,
A
Ascherio,
GA
Colditz,
D
Spiegelman,
MJ
Stampfer.
Smoking and risk of total and fatal prostate cancer in United States health professionals. Cancer Epidemiol Biomarkers Prevention.
1999;
8
:
277-82
.
-
DT
Silverman,
JA
Dunn,
RN
Hoover,
M
Schiffman,
KD
Lillemoe,
JB
Schoenberg.
Cigarette smoking and pancreas cancer: a case-control study based on direct interviews. Journal of the National Cancer Institute.
1994;
86
:
1510-6
.
View Article PubMed Google Scholar -
F
CS,
C
GA,
S
MJ,
G
EL,
H
DJ,
R
EB.
A prospective study of cigarette smoking and the risk of pancreatic cancer. Archives of internal medicine.
1996;
156
:
2255-60
.
-
S
Iodice,
S
Gandini,
P
Maisonneuve,
AB
Lowenfels.
Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks´ Archives of Surgery.
2008;
393
:
535-45
.
View Article PubMed Google Scholar -
JE
Muscat,
SD
Stellman,
D
Hoffmann,
EL
Wynder.
Smoking and pancreatic cancer in men and women. Cancer Epidemiol Biomarkers Prevention.
1997;
6
:
15-9
.
-
SH
Health UDo.
The health consequences of smoking: a report of the Surgeon General. 2004
.
-
SM
Lynch,
A
Vrieling,
JH
Lubin,
P
Kraft,
JB
Mendelsohn,
P
Hartge.
Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. American Journal of Epidemiology.
2009;
170
:
403-13
.
View Article PubMed PMC Google Scholar -
S
Vida,
L
Richardson,
E
Cardis,
D
Krewski,
M
McBride,
ME
Parent.
Brain tumours and cigarette smoking: analysis of the INTERPHONE Canada case-control study. Environmental Health.
2014;
13
:
55
.
View Article PubMed PMC Google Scholar -
A
Chao,
MJ
Thun,
SJ
Henley,
EJ
Jacobs,
ML
McCullough,
EE
Calle.
Cigarette smoking, use of other tobacco products and stomach cancer mortality in US adults: The Cancer Prevention Study II. International Journal of Cancer.
2002;
101
:
380-9
.
View Article PubMed Google Scholar -
A
Maekawa,
K
Mitsumori.
Spontaneous occurrence and chemical induction of neurogenic tumors in rats-influence of host factors and specificity of chemical structure. Critical Reviews in Toxicology.
1990;
20
:
287-310
.
View Article PubMed Google Scholar -
P
Bogovski,
S
Bogovski.
Animal Species in which N-nitroso compounds induce cancer. International Journal of Cancer.
1981;
27
:
471-4
.
View Article PubMed Google Scholar -
BT
Hawkins,
RD
Egleton,
TP
Davis.
Modulation of cerebral microvascular permeability by endothelial nicotinic acetylcholine receptors. American Journal of Physiology. Heart and Circulatory Physiology.
2005;
289
:
H212-9
.
-
JM
Connelly,
MG
Malkin.
Environmental risk factors for brain tumors. Current Neurology and Neuroscience Reports.
2007;
7
:
208-14
.
View Article PubMed Google Scholar -
T
Zheng,
KP
Cantor,
Y
Zhang,
BC
Chiu,
CF
Lynch.
Risk of brain glioma not associated with cigarette smoking or use of other tobacco products in Iowa. Cancer Epidemiol Biomarkers Prevention.
2001;
10
:
413-4
.
-
S
Preston-Martin,
W
Mack,
BE
Henderson.
Risk factors for gliomas and meningiomas in males in Los Angeles County. Cancer Research.
1989;
49
:
6137-43
.
PubMed Google Scholar -
B
Schlehofer,
I
Hettinger,
P
Ryan,
M
Blettner,
S
Preston-Martin,
J
Little.
Occupational risk factors for low grade and high grade glioma: results from an international case control study of adult brain tumours. International Journal of Cancer.
2005;
113
:
116-25
.
View Article PubMed Google Scholar -
M
Musicco,
G
Filippini,
BM
Bordo,
A
Melotto,
G
Morello,
F
Berrino.
Gliomas and occupational exposure to carcinogens: case-control study. American Journal of Epidemiology.
1982;
116
:
782-90
.
View Article PubMed Google Scholar -
A
Ahlbom,
IL
Navier,
S
Norell,
R
Olin,
B
Spannare.
Nonoccupational risk indicators for astrocytomas in adults. American Journal of Epidemiology.
1986;
124
:
334-7
.
View Article PubMed Google Scholar -
SF
Hurley,
JJ
McNeil,
GA
Donnan,
A
Forbes,
M
Salzberg,
GG
Giles.
Tobacco smoking and alcohol consumption as risk factors for glioma: a case-control study in Melbourne, Australia. Journal of Epidemiology and Community Health.
1996;
50
:
442-6
.
View Article PubMed PMC Google Scholar -
PK
Mills,
S
Preston-Martin,
JF
Annegers,
WL
Beeson,
RL
Phillips,
GE
Fraser.
Risk factors for tumors of the brain and cranial meninges in Seventh-Day Adventists. Neuroepidemiology.
1989;
8
:
266-75
.
View Article PubMed Google Scholar -
CN
Holick,
EL
Giovannucci,
B
Rosner,
MJ
Stampfer,
DS
Michaud.
Prospective study of cigarette smoking and adult glioma: dosage, duration, and latency. Neuro-Oncology.
2007;
9
:
326-34
.
View Article PubMed PMC Google Scholar -
VS
Benson,
K
Pirie,
J
Green,
D
Casabonne,
V
Beral,
CMW
Study.
Lifestyle factors and primary glioma and meningioma tumours in the Million Women Study cohort. British Journal of Cancer.
2008;
99
:
185-90
.
View Article PubMed PMC Google Scholar -
JT
Efird,
GD
Friedman,
S
Sidney,
A
Klatsky,
LA
Habel,
NV
Udaltsova.
The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: cigarette smoking and other lifestyle behaviors. Journal of Neuro-Oncology.
2004;
68
:
57-69
.
View Article PubMed Google Scholar -
SA
Silvera,
AB
Miller,
TE
Rohan.
Cigarette smoking and risk of glioma: a prospective cohort study. International Journal of Cancer.
2006;
118
:
1848-51
.
View Article Google Scholar -
B
Schlehofer,
S
Kunze,
W
Sachsenheimer,
M
Blettner,
D
Niehoff,
J
Wahrendorf.
Occupational risk factors for brain tumors: results from a population-based case-control study in Germany. Cancer Causes & Control.
1990;
1
:
209-15
.
View Article PubMed Google Scholar -
B
Schneider,
H
Pulhorn,
B
Rohrig,
NG
Rainov.
Predisposing conditions and risk factors for development of symptomatic meningioma in adults. Cancer Detection and Prevention.
2005;
29
:
440-7
.
View Article PubMed Google Scholar -
L
Mandelzweig,
I
Novikov,
S
Sadetzki.
Smoking and risk of glioma: a meta-analysis. Cancer Causes & Control.
2009;
20
:
1927-38
.
View Article PubMed Google Scholar -
E
Lee,
J
Grutsch,
V
Persky,
R
Glick,
J
Mendes,
F
Davis.
Association of meningioma with reproductive factors. International Journal of Cancer.
2006;
119
:
1152-7
.
View Article PubMed Google Scholar -
S
Preston-Martin,
W
Mack.
Gliomas and meningiomas in men in Los Angeles County: investigation of exposures to N-nitroso compounds. IARC Scientific Publications.
1991;
:
197-203
.
PubMed Google Scholar -
P
Ryan,
MW
Lee,
B
North,
AJ
McMichael.
Risk factors for tumors of the brain and meninges: results from the Adelaide Adult Brain Tumor Study. International Journal of Cancer.
1992;
51
:
20-7
.
View Article PubMed Google Scholar -
D
Wenbin,
C
Zhuo,
M
Zhibing,
Z
Chen,
Y
Ruifan,
J
Jie.
The effect of smoking on the risk of gallbladder cancer: a meta-analysis of observational studies. European Journal of Gastroenterology & Hepatology.
2013;
25
:
373-9
.
View Article PubMed Google Scholar -
D
Aune,
LJ
Vatten,
P
Boffetta.
Tobacco smoking and the risk of gallbladder disease. 2016
.
-
S
Kono,
K
Shinchi,
N
Ikeda,
F
Yanai,
K
Imanishi.
Prevalence of gallstone disease in relation to smoking, alcohol use, obesity, and glucose tolerance: a study of self-defense officials in Japan. American Journal of Epidemiology.
1992;
136
:
787-94
.
View Article PubMed Google Scholar -
M
Okamoto,
Z
Yamagata,
Y
Takeda,
Y
Yoda,
K
Kobayashi,
MA
Fujino.
The relationship between gallbladder disease and smoking and drinking habits in middle-aged Japanese. Journal of Gastroenterology.
2002;
37
:
455-62
.
View Article PubMed Google Scholar -
W
Kratzer,
V
Kachele,
RA
Mason,
R
Muche,
B
Hay,
M
Wiesneth.
Gallstone prevalence in relation to smoking, alcohol, coffee consumption, and nutrition. The Ulm Gallstone Study. Scandinavian Journal of Gastroenterology.
1997;
32
:
953-8
.
-
H
Pastides,
A
Tzonou,
D
Trichopoulos,
K
Katsouyanni,
A
Trichopoulou,
N
Kefalogiannis.
A case-control study of the relationship between smoking, diet, and gallbladder disease. Archives of Internal Medicine.
1990;
150
:
1409-12
.
View Article PubMed Google Scholar -
AJ
McMichael,
PA
Baghurst,
RK
Scragg.
A case-control study of smoking and gallbladder disease: importance of examining time relations. Epidemiology (Cambridge, Mass.).
1992;
3
:
519-22
.
-
I
Kato,
K
Kato,
S
Akai,
S
Tominaga.
A case-control study of gallstones: a major risk factor for biliary tract cancer. Japanese Journal of Cancer Research.
1990;
81
:
578-83
.
View Article PubMed Google Scholar -
SM
Bernard,
RA
Cartwright,
CM
Darwin,
ID
Richards,
B
Roberts,
C
OB’rien.
Hodgkins´ disease: case control epidemiological study in Yorkshire. British Journal of Cancer.
1987;
55
:
85-90
.
View Article PubMed PMC Google Scholar -
GR
Newell,
W
Rawlings,
BK
Kinnear,
P
Correa,
BE
Henderson,
R
Dworsky.
Case-control study of Hodgkins´ disease. I. Results of the interview questionnaire. Journal of the National Cancer Institute.
1973;
51
:
1437-41
.
PubMed Google Scholar -
A
Monnereau,
L
Orsi,
X
Troussard,
C
Berthou,
P
Fenaux,
P
Soubeyran.
Cigarette smoking, alcohol drinking, and risk of lymphoid neoplasms: results of a French case-control study. Cancer Causes & Control.
2008;
19
:
1147-60
.
View Article PubMed Google Scholar -
H
Hjalgrim,
K
Ekstrom-Smedby,
K
Rostgaard,
RM
Amini,
D
Molin,
S
Hamilton-Dutoit.
Cigarette smoking and risk of Hodgkin lymphoma: a population-based case-control study. Cancer.
2007;
Epidemiology
:
Biomarkers & Prevention 16
.
View Article PubMed Google Scholar -
P
Fernberg,
A
Odenbro,
R
Bellocco,
P
Boffetta,
Y
Pawitan,
J
Adami.
Tobacco use, body mass index and the risk of malignant lymphomas-a nationwide cohort study in Sweden. International Journal of Cancer.
2006;
118
:
2298-302
.
View Article PubMed Google Scholar -
NC
Briggs,
HI
Hall,
EA
Brann,
CJ
Moriarty,
RS
Levine.
Cigarette smoking and risk of Hodgkins´ disease: a population-based case-control study. American Journal of Epidemiology.
2002;
156
:
1011-20
.
View Article PubMed Google Scholar -
ET
Chang,
T
Zheng,
EG
Weir,
M
Borowitz,
RB
Mann,
D
Spiegelman.
Childhood social environment and Hodgkins´ lymphoma: new findings from a population-based case-control study. Cancer Epidemiol Biomarkers Prevention.
2004;
13
:
1361-70
.
-
A
Nieters,
S
Rohrmann,
N
Becker,
J
Linseisen,
T
Ruediger,
K
Overvad.
Smoking and lymphoma risk in the European prospective investigation into cancer and nutrition. American Journal of Epidemiology.
2008;
167
:
1081-9
.
View Article PubMed Google Scholar -
JJ
Castillo,
S
Dalia,
H
Shum.
Meta-analysis of the association between cigarette smoking and incidence of Hodgkins´ Lymphoma. Journal of Clinical Oncology.
2011;
29
:
3900-6
.
View Article PubMed Google Scholar -
TN
Sergentanis,
P
Kanavidis,
T
Michelakos,
ET
Petridou.
Cigarette smoking and risk of lymphoma in adults: a comprehensive meta-analysis on Hodgkin and non-Hodgkin disease. European Journal of Cancer Prevention.
2013;
22
:
131-50
.
View Article Google Scholar -
M
Kamper-J?rgensen,
K
Rostgaard,
SL
Glaser,
SH
Zahm,
W
Cozen,
KE
Smedby.
Cigarette smoking and risk of Hodgkin lymphoma and its subtypes: a pooled analysis from the International Lymphoma Epidemiology Consortium (InterLymph). Annals of Oncology : Official Journal of the European Society for Medical Oncology.
2013;
24
:
2245-55
.
-
E
Stagnaro,
V
Ramazzotti,
P
Crosignani,
A
Fontana,
G
Masala,
L
Miligi.
Smoking and hematolymphopoietic malignancies. Cancer Causes & Control.
2001;
12
:
325-34
.
View Article PubMed Google Scholar -
S
Franceschi,
D
Serraino,
E
Bidoli,
R
Talamini,
U
Tirelli,
A
Carbone.
The epidemiology of non-Hodgkins´ lymphoma in the north-east of Italy: a hospital-based case-control study. Leukemia Research.
1989;
13
:
465-72
.
View Article Google Scholar -
RA
Nelson,
AM
Levine,
G
Marks,
L
Bernstein.
Alcohol, tobacco and recreational drug use and the risk of non-Hodgkins´ lymphoma. British Journal of Cancer.
1997;
76
:
1532-7
.
View Article PubMed PMC Google Scholar -
E
Stagnaro,
R
Tumino,
S
Parodi,
P
Crosignani,
A
Fontana,
G
Masala.
Non-Hodgkins´ lymphoma and type of tobacco smoke. Cancer Epidemiol Biomarkers Prevention.
2004;
13
:
431-7
.
-
JC
Schroeder,
AF
Olshan,
R
Baric,
GA
Dent,
CR
Weinberg,
B
Yount.
A case-control study of tobacco use and other non-occupational risk factors for t(14;18) subtypes of non-Hodgkins´ lymphoma (United States). Cancer Causes & Control.
2002;
13
:
159-68
.
View Article PubMed Google Scholar -
DS
Freedman,
PE
Tolbert,
R
Coates,
EA
Brann,
CR
Kjeldsberg.
Relation of cigarette smoking to non-Hodgkins´ lymphoma among middle-aged men. American Journal of Epidemiology.
1998;
148
:
833-41
.
View Article PubMed Google Scholar -
P
Fabbro-Peray,
JP
Daures,
JF
Rossi.
Environmental risk factors for non-Hodgkins´ lymphoma: a population-based case-control study in Languedoc-Roussillon, France. Cancer Causes & Control.
2001;
12
:
201-12
.
View Article PubMed Google Scholar -
AM
Hughes,
BK
Armstrong,
CM
Vajdic,
J
Turner,
A
Grulich,
L
Fritschi.
Pigmentary characteristics, sun sensitivity and non-Hodgkin lymphoma. International Journal of Cancer.
2004;
110
:
429-34
.
View Article PubMed Google Scholar -
AS
Parker,
JR
Cerhan,
F
Dick,
J
Kemp,
TM
Habermann,
RB
Wallace.
Smoking and risk of non-Hodgkin lymphoma subtypes in a cohort of older women. Leukemia & Lymphoma.
2000;
37
:
341-9
.
PubMed Google Scholar -
H
Besson,
P
Renaudier,
RM
Merrill,
B
Coiffier,
C
Sebban,
J
Fabry.
Smoking and non- Hodgkins´ lymphoma: a case-control study in the Rhone-Alpes region of France. Cancer Causes & Control.
2003;
14
:
381-9
.
View Article PubMed Google Scholar -
LM
Morton,
P
Hartge,
TR
Holford,
EA
Holly,
BC
Chiu,
P
Vineis.
Cigarette smoking and risk of non-Hodgkin lymphoma: a pooled analysis from the International Lymphoma Epidemiology Consortium (interlymph). Cancer.
2005;
Epidemiology
:
Biomarkers & Prevention 14
.
View Article PubMed Google Scholar -
C
Schollkopf,
KE
Smedby,
H
Hjalgrim,
K
Rostgaard,
O
Gadeberg,
G
Roos.
Cigarette smoking and risk of non-Hodgkins´ lymphoma-a population-based case-control study. Cancer.
2005;
Epidemiology
:
Biomarkers & Prevention 14
.
View Article PubMed Google Scholar -
WR
Diver,
AV
Patel,
MJ
Thun,
LR
Teras,
SM
Gapstur.
The association between cigarette smoking and non-Hodgkin lymphoid neoplasms in a large US cohort study. Cancer Causes & Control.
2012;
23
:
1231-40
.
View Article PubMed Google Scholar -
LM
Morton,
TR
Holford,
B
Leaderer,
P
Boyle,
SH
Zahm,
Y
Zhang.
Cigarette smoking and risk of non-Hodgkin lymphoma subtypes among women. British Journal of Cancer.
2003;
89
:
2087-92
.
View Article PubMed PMC Google Scholar -
WR
Diver,
LR
Teras,
MM
Gaudet,
SM
Gapstur.
Exposure to environmental tobacco smoke and risk of non-Hodgkin lymphoma in nonsmoking men and women. American Journal of Epidemiology.
2014;
179
:
987-95
.
View Article PubMed Google Scholar -
EE
Calle,
C
Rodriguez,
EJ
Jacobs,
ML
Almon,
A
Chao,
ML
McCullough.
The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer.
2002;
94
:
2490-501
.
View Article Google Scholar -
E
Nawar,
SM
Mbulaiteye,
JE
Gallant,
DA
Wohl,
M
Ardini,
TH
Risk factors for Kaposis´ sarcoma among HHV-8 seropositive homosexual men with AIDS. International Journal of Cancer.
2005;
115
:
296-300
.
View Article PubMed Google Scholar -
F
Song,
AA
Qureshi,
X
Gao,
T
Li,
J
Han.
Smoking and risk of skin cancer: a prospective analysis and a meta-analysis. International Journal of Epidemiology.
2012;
41
:
1694-705
.
View Article PubMed PMC Google Scholar -
LA
Anderson,
C
Lauria,
N
Romano,
EE
Brown,
D
Whitby,
BI
Graubard.
Risk factors for classical Kaposi sarcoma in a population-based case-control study in Sicily. Cancer.
2008;
Epidemiology
:
Biomarkers & Prevention 17
.
View Article PubMed PMC Google Scholar -
PS
Moore,
Y
Chang.
Kaposis´ sarcoma (KS), KS-associated herpesvirus, and the criteria for causality in the age of molecular biology. American Journal of Epidemiology.
1998;
147
:
217-21
.
View Article PubMed Google Scholar -
PL
Rady,
A
Yen,
RW
Martin,
I
Nedelcu,
TK
Hughes,
SK
Tyring.
Herpesvirus-like DNA sequences in classic Kaposis´ sarcomas. Journal of Medical Virology.
1995;
47
:
179-83
.
View Article PubMed Google Scholar -
EE
Brown,
D
Fallin,
I
Ruczinski,
A
Hutchinson,
B
Staats,
F
Vitale.
Associations of classic Kaposi sarcoma with common variants in genes that modulate host immunity. Cancer.
2006;
Epidemiology
:
Biomarkers & Prevention 15
.
View Article PubMed Google Scholar -
D
Whitby,
M
Luppi,
C
Sabin,
P
Barozzi,
ARD
Biase,
F
Balli.
Detection of antibodies to human herpesvirus 8 in Italian children: evidence for horizontal transmission. British Journal of Cancer.
2000;
82
:
702-4
.
View Article PubMed PMC Google Scholar -
DR
Hoover,
C
Black,
LP
Jacobson,
O
Martinez-Maza,
D
Seminara,
A
Saah.
Epidemiologic analysis of Kaposis´ sarcoma as an early and later AIDS outcome in homosexual men. American Journal of Epidemiology.
1993;
138
:
266-78
.
View Article PubMed Google Scholar -
JJ
Goedert,
BM
Scoppio,
R
Pfeiffer,
L
Neve,
AB
Federici,
LR
Long.
Treatment of classic Kaposi sarcoma with a nicotine dermal patch: a phase II clinical trial. Journal of the European Academy of Dermatology and Venereology.
2008;
22
:
1101-9
.
View Article PubMed Google Scholar -
J
Chang,
R
Renne,
D
Dittmer,
D
Ganem.
Inflammatory cytokines and the reactivation of Kaposis´ sarcoma-associated herpesvirus lytic replication. Virology.
2000;
266
:
17-25
.
View Article PubMed Google Scholar -
E
Guttman-Yassky,
J
Dubnov,
Z
Kra-Oz,
R
Friedman-Birnbaum,
M
Silbermann,
M
Barchana.
Classic Kaposi sarcoma. Which KSHV-seropositive individuals are at risk?. Cancer.
2006;
106
:
413-9
.
-
LJ
Conley,
TJ
Bush,
SP
Buchbinder,
KA
Penley,
FN
Judson,
SD
Holmberg.
The association between cigarette smoking and selected HIV-related medical conditions. AIDS (London, England).
1996;
10
:
1121-6
.
PubMed Google Scholar -
B
Secretan,
K
Straif,
R
Baan,
Y
Grosse,
FE
Ghissassi,
V
Bouvard.
A review of humancarcinogens–Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. 2009
.
-
CA
Wensveen,
MT
Bastiaens,
CJ
Kielich,
MJ
Berkhout,
RG
Westendorp,
BJ
Vermeer,
SLS
Cancer.
Relation between smoking and skin cancer. Journal of Clinical Oncology.
2001;
19
:
231-8
.
View Article PubMed Google Scholar -
A
Odenbro,
P
Gillgren,
R
Bellocco,
P
Boffetta,
N
Hakansson,
J
Adami.
The risk for cutaneous malignant melanoma, melanoma in situ and intraocular malignant melanoma in relation to tobacco use and body mass index. British Journal of Dermatology.
2007;
156
:
99-105
.
View Article PubMed Google Scholar -
AS
Boyd,
Y
Shyr,
LE
King.
Basal cell carcinoma in young women: an evaluation of the association of tanning bed use and smoking. Journal of the American Academy of Dermatology.
2002;
46
:
706-9
.
View Article PubMed Google Scholar -
DM
Freedman,
A
Sigurdson,
MM
Doody,
K
Mabuchi,
MS
Linet.
Risk of basal cell carcinoma in relation to alcohol intake and smoking. Cancer Epidemiol Biomarkers Prevention.
2003;
12
:
1540-3
.
-
MC
Kessides,
L
Wheless,
J
Hoffman-Bolton,
S
Clipp,
RM
Alani,
AJ
Alberg.
Cigarette smoking and malignant melanoma: a case-control study. Journal of the American Academy of Dermatology.
2011;
64
:
84-90
.
View Article PubMed PMC Google Scholar -
JO
DeLancey,
LM
Hannan,
SM
Gapstur,
MJ
Thun.
Cigarette smoking and the risk of incident and fatal melanoma in a large prospective cohort study. Cancer Causes & Control.
2011;
22
:
937-42
.
View Article PubMed Google Scholar -
VB
Yerger,
RE
Malone.
Melanin and nicotine: A review of the literature. Nicotine & Tobacco Research.
2006;
8
:
487-98
.
View Article PubMed Google Scholar -
CM
Mills,
SA
Hill,
R
Marks.
Transdermal nicotine suppresses cutaneous inflammation. Archives of Dermatology.
1997;
133
:
823-5
.
View Article PubMed Google Scholar -
CM
Mills,
SA
Hill,
R
Marks.
Altered inflammatory responses in smokers. BMJ (Clinical Research Ed.).
1993;
307
:
911-2
.
-
DM
Freedman,
A
Sigurdson,
MM
Doody,
RS
Rao,
MS
Linet.
Risk of melanoma in relation to smoking, alcohol intake, and other factors in a large occupational cohort. Cancer Causes & Control.
2003;
14
:
847-57
.
View Article PubMed Google Scholar -
A
Hallquist,
L
Hardell,
A
Degerman,
L
Boquist.
Occupational exposures and thyroid cancer: results of a case-control study. European Journal of Cancer Prevention.
1993;
2
:
345-9
.
View Article Google Scholar -
MR
Galanti,
L
Hansson,
E
Lund,
R
Bergstrom,
L
Grimelius,
H
Stalsberg.
Reproductive history and cigarette smoking as risk factors for thyroid cancer in women: a population-based case-control study. Cancer Epidemiol Biomarkers Prevention.
1996;
5
:
425-31
.
-
N
Kreiger,
R
Parkes.
Cigarette smoking and the risk of thyroid cancer. European Journal of Cancer.
2000;
(Oxford
:
England) 36
.
View Article Google Scholar -
SH
Jee,
JM
Samet,
H
Ohrr,
JH
Kim,
IS
Kim.
Smoking and cancer risk in Korean men and women. Cancer Causes & Control.
2004;
15
:
341-8
.
View Article PubMed Google Scholar -
CL
Meinhold,
E
Ron,
SJ
Schonfeld,
BH
Alexander,
DM
Freedman,
MS
Linet.
Nonradiation risk factors for thyroid cancer in the US Radiologic Technologists Study. American Journal of Epidemiology.
2010;
171
:
242-52
.
View Article PubMed PMC Google Scholar -
C
Iribarren,
T
Haselkorn,
IS
Tekawa,
GD
Friedman.
Cohort study of thyroid cancer in a San Francisco Bay area population. International Journal of Cancer.
2001;
93
:
745-50
.
View Article PubMed Google Scholar -
WJ
Mack,
S
Preston-Martin,
L
Bernstein,
D
Qian.
Lifestyle and other risk factors for thyroid cancer in Los Angeles County females. Annals of Epidemiology.
2002;
12
:
395-401
.
View Article Google Scholar -
V
Zivaljevic,
H
Vlajinac,
J
Marinkovic,
I
Paunovic,
A
Diklic,
R
Dzodic.
Cigarette smoking as a risk factor for cancer of the thyroid in women. Tumori.
2004;
90
:
273-5
.
View Article PubMed Google Scholar -
CM
Kitahara,
MS
Linet,
LEB
Freeman,
DP
Check,
TR
Church,
Y
Park.
Cigarette smoking, alcohol intake, and thyroid cancer risk: a pooled analysis of five prospective studies in the United States. Cancer Causes & Control.
2012;
23
:
1615-24
.
View Article PubMed PMC Google Scholar -
WJ
Mack,
S
Preston-Martin,
LD
Maso,
R
Galanti,
M
Xiang,
S
Franceschi.
A pooled analysis of case-control studies of thyroid cancer: cigarette smoking and consumption of alcohol, coffee, and tea. Cancer Causes & Control.
2003;
14
:
773-85
.
View Article PubMed Google Scholar -
RM
Belin,
BC
Astor,
NR
Powe,
PW
Ladenson.
Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III). The Journal of Clinical Endocrinology and Metabolism.
2004;
89
:
6077-86
.
View Article Google Scholar -
OP
Soldin,
BE
Goughenour,
SZ
Gilbert,
HJ
Landy,
SJ
Soldin.
Thyroid hormone levels associated with active and passive cigarette smoking. Thyroid.
2009;
19
:
817-23
.
View Article PubMed PMC Google Scholar -
K
Boelaert,
J
Horacek,
RL
Holder,
JC
Watkinson,
MC
Sheppard,
JA
Franklyn.
Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. The Journal of Clinical Endocrinology and Metabolism.
2006;
91
:
4295-301
.
View Article PubMed Google Scholar -
A
Kumar,
CM
Klinge,
RE
Goldstein.
Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. International Journal of Oncology.
2010;
36
:
1067-80
.
PubMed Google Scholar -
MS
Shiels,
S
Rohrmann,
A
Menke,
E
Selvin,
CJ
Crespo,
N
Rifai.
Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes & Control.
2009;
20
:
877-86
.
View Article PubMed PMC Google Scholar
Comments

Downloads
Article Details
Volume & Issue : Vol 5 No 4 (2018)
Page No.: 2142-2159
Published on: 2018-04-16
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 33389 times
- Download PDF downloaded - 4025 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress