Abstract
Background: The congenital hypothyroidism (CH) is one of the most common congenital endocrine disorders as well as the most preventable mental retardation cause and body growth disorder. Several factors such as therapeutic interventions might affect the infants’ growth status. The current study was aimed to evaluate the therapeutic interventions’ effect on growth pattern of infants with congenital hypothyroidism in Yazd.
Methods: This retrospective cohort study was performed on all neonates with CH, who were born during years 2006 and 2008. The effectiveness of therapeutic interventions indices including the age of treatment onset, the administered dosage of Levothyroxine and normalization time of T4 and TSH level on growth pattern of children with CH by measuring height, weight and head circumference during the first five years was evaluated.
Results: The results of the current study showed a significant increase in height, weight, and head circumference in infants from early infancy to 60 months of age as well as an ascending trend of the aforementioned variables(P<0.05).
Conclusion: The significant effect of therapeutic interventions such as the age of treatment onset less than 30 days, normalization time of TSH during 30 days after diagnosing and normalization age of T4 less than 14 days was reported on height and weight growths of patients with congenital hypothyroidism. Also, the trend of head circumference growth would be increased in patients equally.
Background
Height, weight, and head circumference size are considered as one of the best indicators of infants’ general health. The abnormal growth may indicate the presence of a disease and/or a disorder in apparently normal infants. The congenital hypothyroidism is one of the causes of growth disorder in infants [1][2]. The congenital hypothyroidism was described as the lack of thyroid hormone in birth time reported as one of the most common congenital endocrine disorders as well as the most preventable causes of mental retardation and body growth disorders [3][4]. The incidence of congenital hypothyroidism was substantially increased in recent years with ratio of 1:1400 to 1:2800 [4]. According to the studies performed in Iran, the incidence of the aforementioned disorder was increased from the ratio of 1:1400 to 1:1900 being statistically higher than the global average [5]. A meta-analysis study reported the incidence of newborns’ congenital hypothyroidism with the ratio of 2:1000 in Iran [6].
The studies reported the prevalence of congenital hypothyroidism in different ranges in the world being 7.14 in Nigeria to 14 per 1000 births in Japan [3][7]. The reports stated the higher incidence of congenital hypothyroidism in Iran than other regions of the world before and after applying the congenital hypothyroidism screening program [8][9]. in this regard, the national studies have shown various incidence of congenital hypothyroidism in Iran with the ratio of 1:1000, 1:370, 1:1433, 1:400 and 1:1608 live births in Tehran, Isfahan, Shiraz, Kurdistan, and Yazd, respectively [10]–[13]. Nowadays, the infants’ screening program application leads to the early diagnosis of patients with various genetic and/or congenital diseases such as congenital hypothyroidism and accordingly applying the suitable treatment with Levothyroxine alternative dosage administration suppressing its adverse outcomes [14][15]. It has been accepted that hypothyroidism in childhood, in spite of proper treatment, can disrupt growth in adulthood; however, many studies have reported that children with congenital hypothyroidism are normal from the onset of infancy by screening programs and treatment [16].
Some authors believe that several factors including age of treatment onset, utilized therapeutic dosage, Thyroid Stimulating Hormone (TSH) normalization age and the severity of disorder might affect the growth status of infants with congenital hypothyroidism. The effect of aforementioned factors on growth pattern in infants with congenital hypothyroidism undergone diagnosis through screening and early treatment in the onset of infancy was remained controversial [16]–[18]. The studies demonstrated a normal pattern of body growth and height in adulthood in infants with congenital hypothyroidism using the continuous treatment [17][18].
Furthermore, the thyroid’s normal function plays an important role in brain development prognosis. The patients that their T4 level becomes normal during 2 or more weeks after onset of treatment significantly acquired a lower mark of brain development than whom reach to normal level of T4 during 1 week after onset of treatment [19]. On the other hand, the several authors reported a more severe pattern of brain development disorder in patients, undergone late treatment and low dosage of Levothyroxine [19]–[21].
In a study performed to evaluate the various effects of early dosages of Levothyroxine on the growth pattern of patients with congenital hypothyroidism, there wasn’t significant difference between weight, height, and head circumference size of the patients groups, but the growth mean of them was near to the normal growth mean basis on age. Although beginning of treatment with high dosage of Levothyroxine immediately normalized the TSH serum level during a month and leaded to Intelligence Quotient improvement in 4-year-old even in patients with severe congenital hypothyroidism in the time of diagnosis, it couldn’t affect their growth [22].
The findings of several studies that have performed to evaluate the growth pattern of infants with congenital hypothyroidism and related factors were various and controversial. Some studies indicated the findings portraying delaying of growth in infants with congenital hypothyroidism, but the early treatment leads to their normal growth pattern. Conversely, some studies reported no significant difference in growth pattern between the infants with congenital hypothyroidism and healthy ones [20,23]–[25].
Few studies performed to evaluate the therapeutic interventions effects and the related factors on growth pattern of the infants with congenital hypothyroidism in Iran, to our knowledge, and most of them assessed the patients’ mental disorders [11],[26]. Therefore, given that the physical problems associated with this disease are as important as the mental disabilities that it is producing and affect the infants’ future life. Also, considering the high incidence of the congenital hypothyroidism in Iran, the current study was conducted to evaluate the therapeutic interventions’ effect on growth pattern of infants with congenital hypothyroidism in Yazd.
With the exception of Hashemipour’s study in Isfahan, such a study has not been conducted in Iran and Yazd province so far. As regard very high prevalence of this disease in Iran, especially in Yazd and also implementation of the neonatal screening program in this city for many years which lead to early diagnosis and treatment interventions, therefore, this study was conducted with the aim of evaluation of the therapeutic interventions’ effects on the growth pattern of infants with congenital hypothyroidism in Yazd city. Consequently, timely and proper implementations of screening programs and treatment interventions could be done by comparing the pattern of physical growth of children in terms of the therapeutic factors taken by specialist physicians and sending these results to doctors, healthcare policy officers and healthcare professionals.
Methods
Design and implementation method of study
The current study was designed as a retrospective cohort and carried out on all of the newborns with congenital hypothyroidism diagnosed through screening program and undergone treatment. All of the newborns from 2007 to 2009 with hypothyroidism were diagnosed through congenital hypothyroidism screening program and undergone treatment, were enrolled to the study after meeting the inclusion and exclusion criteria; afterwards, the effect of related-indices to the therapeutic interventions were assessed by physicians on growth pattern of height, weight, and head circumference size of the infants with congenital hypothyroidism including the age of treatment onset, the administered first dosage of Levothyroxine and normalization period of serum T4 and TSH.
The inclusion criteria was all of the Yazd newborns diagnosed with congenital hypothyroidism through screening program and referred to the healthcare center of Yazd to receive the healthcare services. The exclusion criteria was all of the newborns of other regions diagnosed with congenital hypothyroidism together with another disease including pre-mature newborns, intrauterine infants with severe anomaly. Moreover, the lack of knowledge related to study’s main variables was considered as exclusion criteria; for instance, undetermined age, and anthropometric variables or apparent error in measurements.
Screening program, diagnosis and treatment
The abnormal values based on screening function to diagnose congenital hypothyroidism were included as TSH> 10 mU/L and T4< 6.5 µg/dl. The anthropometric indices were measured from early infancy to 1 year of age every 3 months, from 1 to 2 year(s) of age every 6 months, and 2 to over-2 years of age annually. In addition, height and weight as well as head circumference size were measured to 5 and 2 years of age, respectively. Therefore, every infant were evaluated in 10 episodes for height and weight and 7 episodes for head circumference size after enrolling to the study. According to the congenital hypothyroidism screening program of the infants in Iran, The treatment begin was classified based on the newborn’s age as the following:
Less than 28 days (favorable), from 28 to 40 days (acceptable), more than 41 days (unfavorable)
The normalization period of serum T4 and TSH level was categorized as the following based on the aforementioned instruction.
The normalization period of T4 level was classified to two groups as ≤14 and >14 days
after initiating the treatment, respectively. Also, the normalization period of TSH level was classified to two groups as ≤30 and >30 days after treatment begin, respectively. The infants with congenital hypothyroidism were assigned to two groups as ≤25 and >25 µg/day based on the first administered therapeutic dosage.
Generally, a total of 69 patients diagnosed with congenital hypothyroidism. Ultimately 55 patients were enrolled to the study after meeting the inclusion criteria. Then, the effectiveness of therapeutic interventions on growth pattern was assessed.
Data collection
In the present study, after coordination with an expert on congenital hypothyroidism and obtaining the necessary permissions from the provincial health center and in coordination with the doctor responsible for the treatment of affected children, an Excel file related to the information of newborns diagnosed with hypothyroidism from 2006 - 2008. This file included information about the factors of treatment interventions implemented for children with hypothyroidism. Related variables were extracted from this file.
Furthermore, to collect data related to the anthropometric indices and physical growth pattern (variables such as of height, weight, and head circumference) by referring to the form of monitoring and care of newborns in health centers was executed by using data collection form.
Data analysis
The collected data were analyzed using SPSS software version 22. In order to descriptive analyses height, weight, head circumference size means were estimated based on gender, treatment age, the first administered dosage of Levothyroxine and normalization period of serum T4 and TSH level. In addition, independent sample t-test, repeated measures and ANOVA were utilized for analytical analyses.
Ethical consideration
- Obtaining informed consent from the doctor responsible for treatment interventions and parents of patients.
- Data and information collected and published collectively.
- The collected data have been kept confidential.
- Collected data have been used only to achieve the purpose of the study.
Results
The results of the current study were surveyed in two parts including descriptive and analytical analyses. The descriptive results are presented in Table 1 showing 5 year-old growth, height, weight, and head circumference size means of the infants with congenital hypothyroidism based on gender, the administered dosage of Levothyroxine, age of treatment begin, normalization period of T4 and TSH level. The 5 year-old growth mean of height, weight, and head circumference in males was higher than that in the females. Also, the 5 year-old growth means of height, weight, and head circumference in infants, undergone lower dosage of Levothyroxine was greater than that in subjects, undergone higher dosage of Levothyroxine. The 5 year-old growth means of height, weight, and head circumference in infants, undergone treatment in early age was higher than that in the subjects, undergone treatment in late age. Furthermore, the 5 year-old growth means of height, weight, and head circumference in infants with short- term normalization time to adjust serum T4 and TSH was higher than that in the subjects with long-term normalization time.
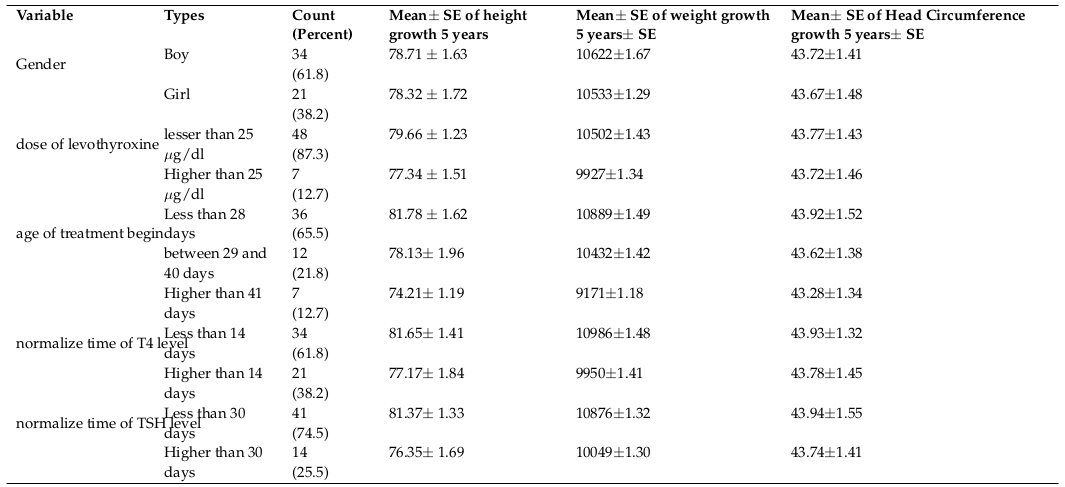
The trend of 5 year-old growth of height was assessed based on the administered dosage of Levothyroxine ( Figure 1 ) showing a significant trend of height growth in infants undergone lower dosage of Levothyroxine being statistically insignificant through using Repeated Measure (P= 0.37).
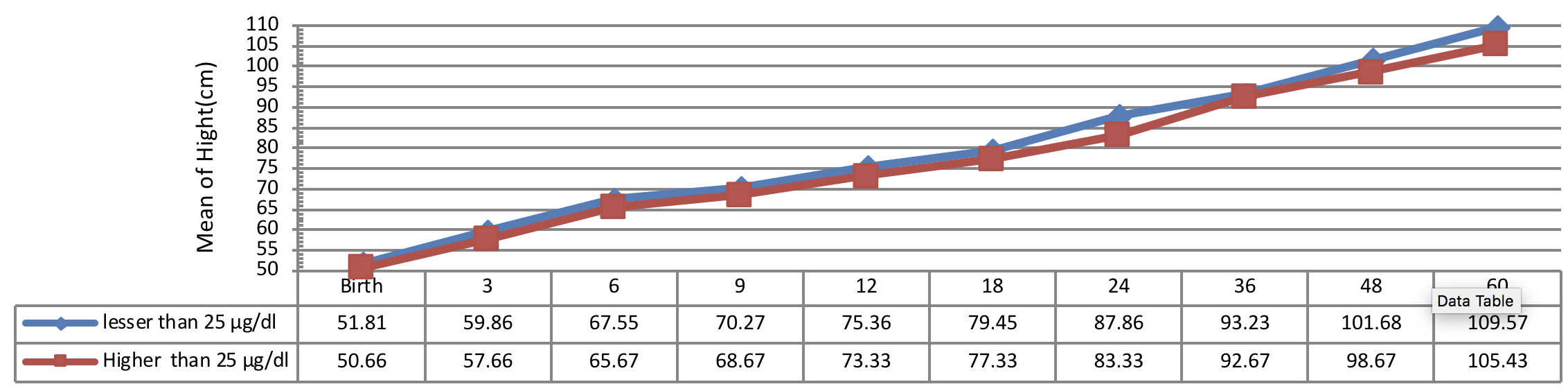
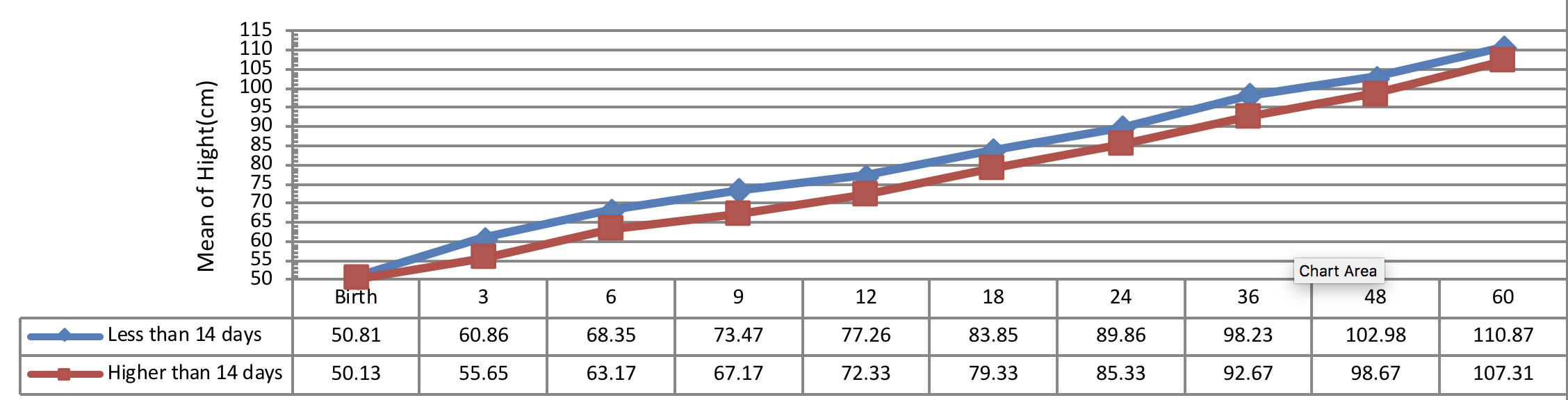
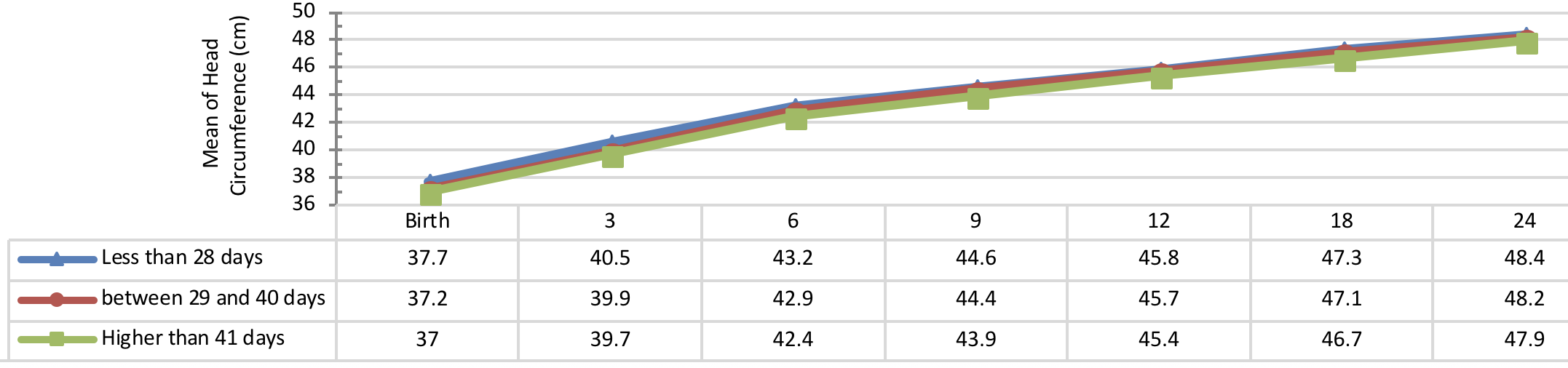
Figure 2 indicates the 5 year-old growth of height in infants with congenital hypothyroidism based on age of treatment begin showing a significant height growth of infants undergone treatment earlier than other subjects in all periods. A statistical significant difference was observed between the height growth of infants, undergone treatment less than 28 days of diagnosis and the subjects, undergone treatment more than 41 days of diagnosis (P< 0.001). Conversely, there was no significant difference between the 5 year-old growth of height in infants, undergone treatment from 28 to 40 days of diagnosis with other infants (P> 0.05).
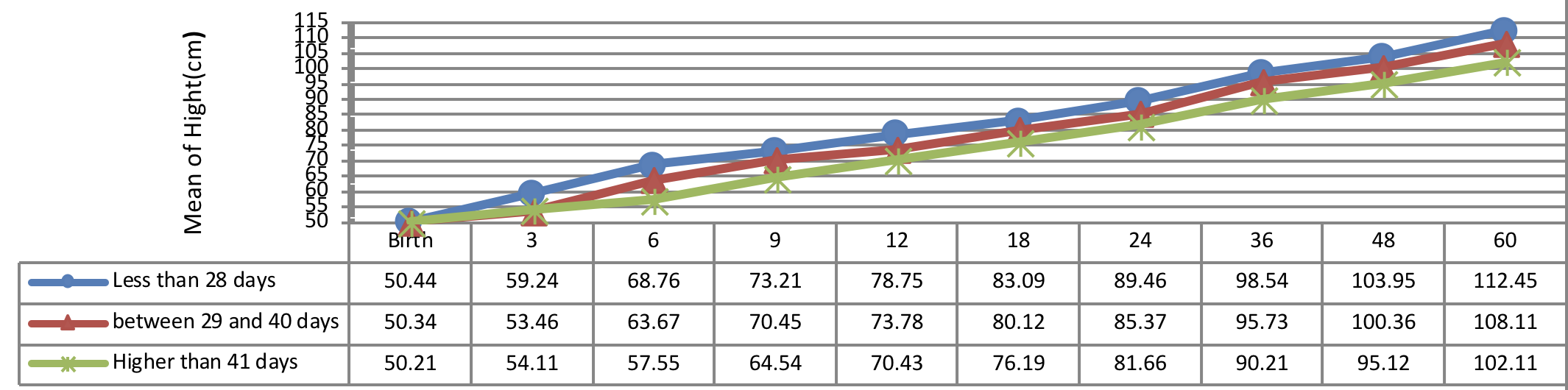
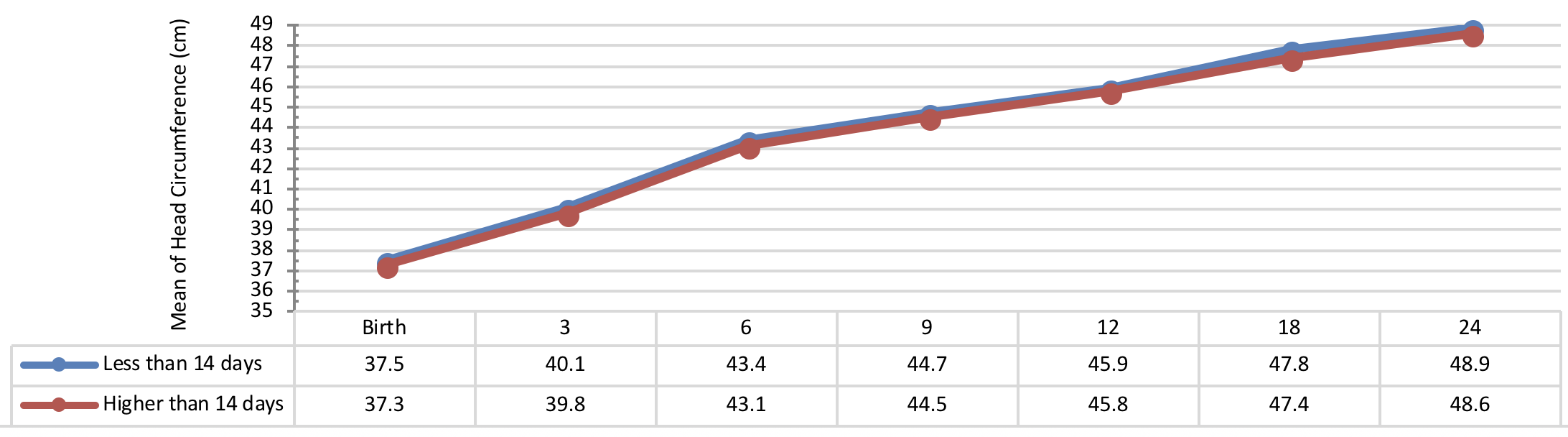
Figure 3 indicates the 5 year-old growth of height in infants with congenital hypothyroidism based on normalization period of serum TSH level showing a better height growth pattern of infants with normalization period during 30 days as compared to the infants with normalization period more than 30 days being statistically significant (P= 0.031).
Figure 4 indicates the 5 year-old growth of height in infants with congenital hypothyroidism based on normalization period of serum T4 level showing a better height growth pattern of infants with normalization period during 14 days as compared to the infants with normalization period more than 14 days being statistically significant (P= 0.028).
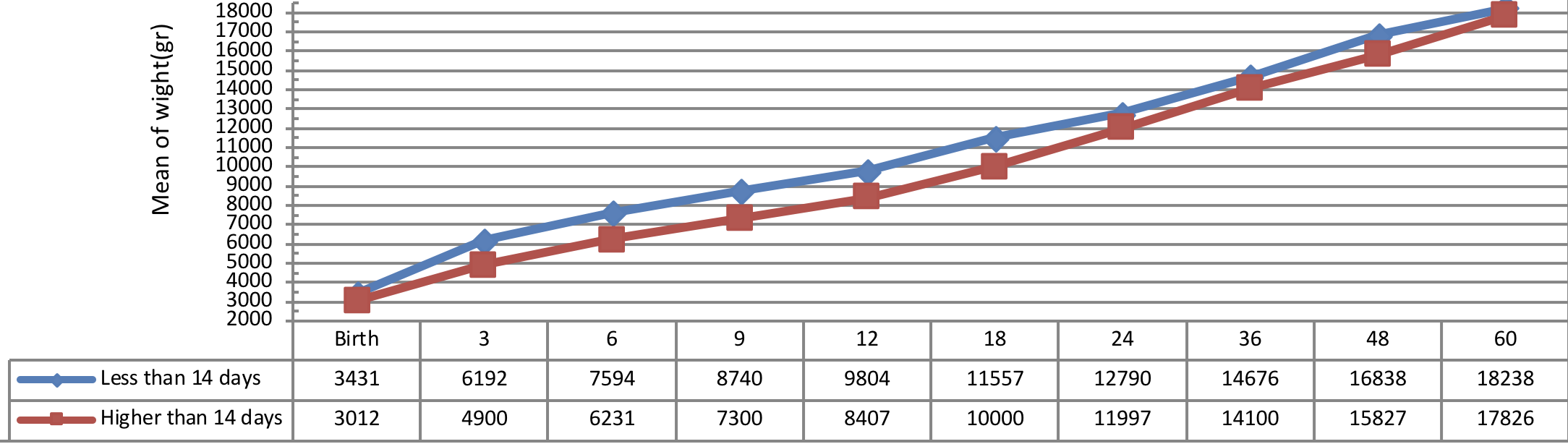
Comparison of weight growth of the infants with congenital hypothyroidism in different periods was presented in Figure 5 , Figure 6 , Figure 7 and Figure 8 based on the therapeutic indices from early infancy to 60 months of age.
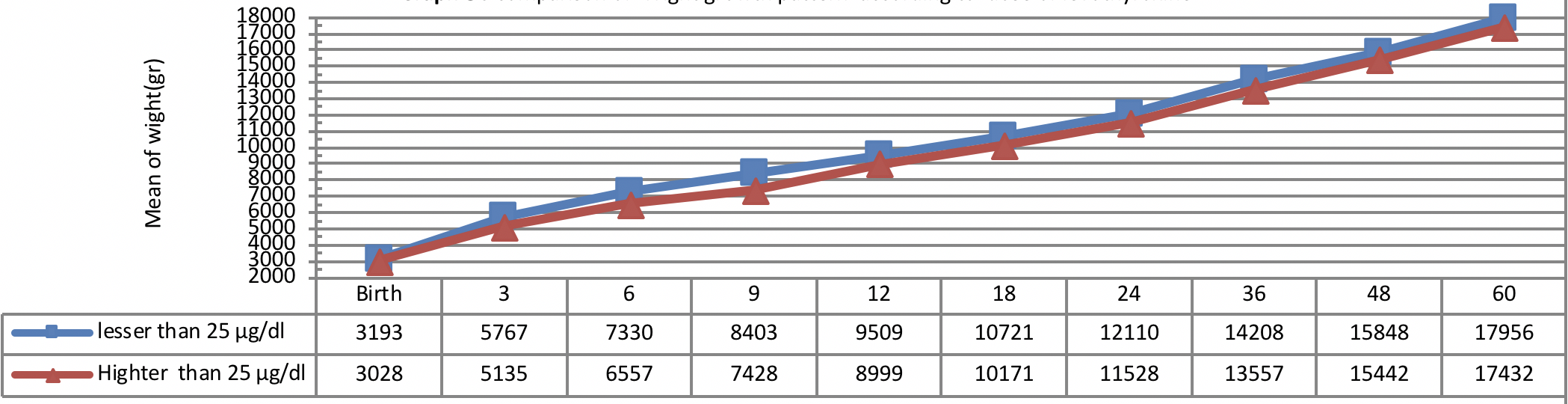
Figure 5 indicates weight growth of infants with congenital hypothyroidism based on the administered dosage of Levothyroxine from early infancy to 60 months of age showing better weight growth pattern of infants with lower dosage of Levothyroxine (≤ 25 µg/day) as compared to the infants with higher dosage of Levothyroxine (>25 µg/day) being statistically insignificant (P=0.33).
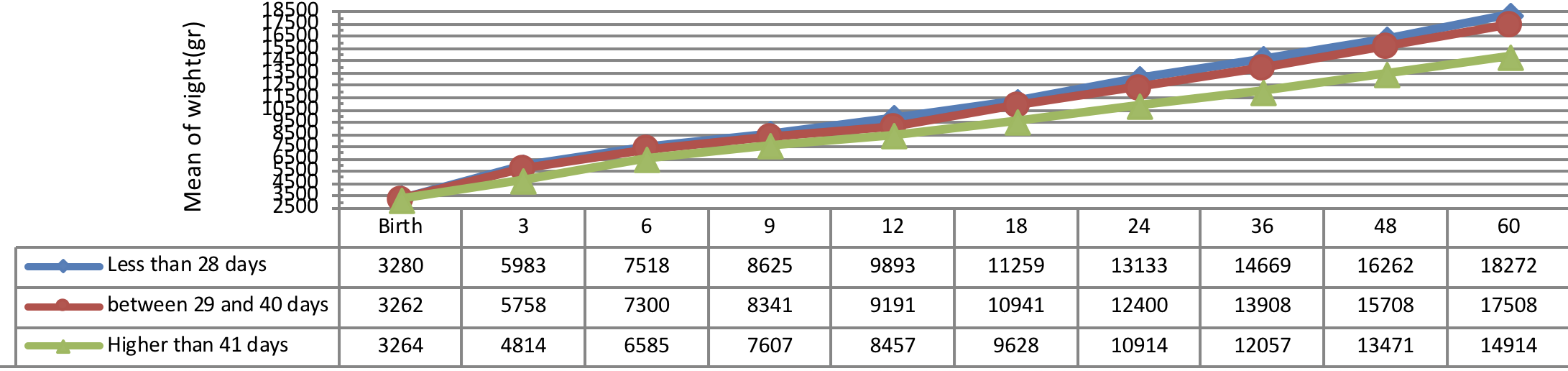
Figure 6 indicates weight growth of infants with congenital hypothyroidism based on the age of treatment begin showing a better weight growth pattern of infants undergone treatment immediately as compared to the infants undergone treatment lately.
The insignificant physical and weight growth was observed in infants, undergone medical treatment lately (41 days after diagnosing). There was a significant difference between the weight growth of infants undergone treatment after 41 days of diagnosis and the subjects undergone 01 treatment before 41 days of diagnosis, but the height growth was not substantial in these infants (P= 0.012).
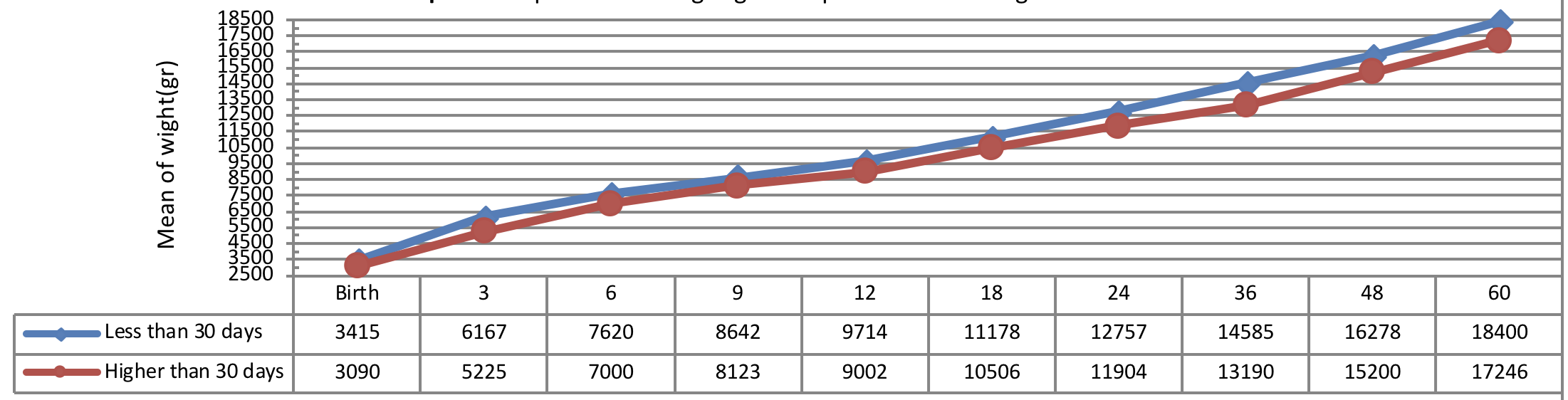
Figure 7 indicates weight growth of infants with congenital hypothyroidism based on the normalization period of serum TSH level showing a more significant weight growth of infants with short-term normalization period (less than 30 days after applying the therapeutic interventions) as compared to the subjects with long-term normalization period being statistically significant (P= 0.041).
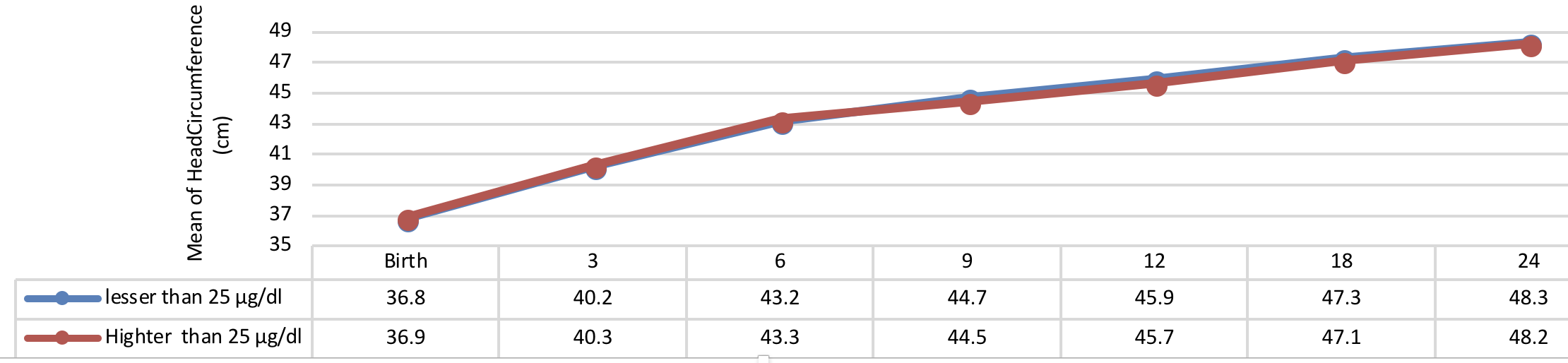
Figure 8 indicates weight growth of infants with congenital hypothyroidism based on the normalization period of serum T4 level showing a more significant weight growth of infants with short-term normalization period (less than 14 days after applying the therapeutic interventions) as compared to the subjects with long-term normalization period (more than 14 days after applying the therapeutic interventions) being statistically significant from early infancy to 24 months of age (P= 0.041), but there was no significant difference between the groups in terms of weight growth trend after 24 months of age (P> 0.05).
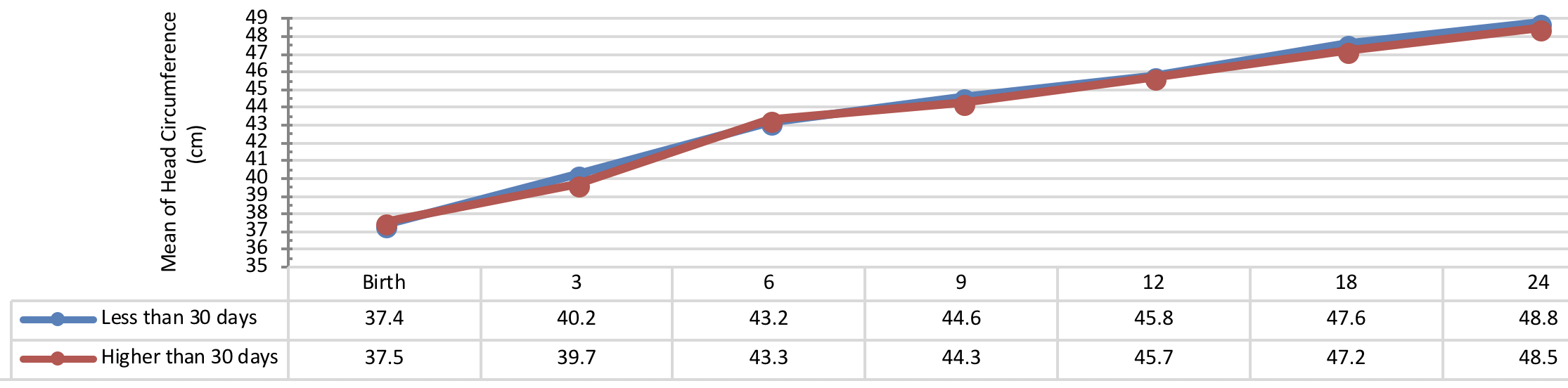
Comparison of head circumference growth of the infants with congenital hypothyroidism in different periods was presented in Figure 9 , Figure 10 , Figure 11 and Figure 12 based on the therapeutic indices from early infancy to 60 months of age. A remarkable similarity in head circumference development was observed between the infants. Therefore, there was no statistical significant difference between the head circumference growth in infants (P> 0.05).
Discussion
The current study was designed as a longitudinal retrospective cohort performed on a total of 55 infants with congenital hypothyroidism diagnosed through the screening program undergone treatment afterwards. They were evaluated in terms of 5 year-old growth of height, weight, and head circumference based on the therapeutic interventions indices including age of treatment begin, the administered dosage of Levothyroxine and normalization time of T4 and TSH level. The results of the current study showed a significant increase in height, weight, and head circumference in infants from early infancy to 60 months of age as well as an ascending trend of the aforementioned variables.
Too many challenges were proposed for administering the necessary dosage of Levothyroxine as well as the normal level of TSH in recent years [27]. The effect of medication dosage and time of alternative treatment onset on newborns with congenital hypothyroidism has been always controversial. The European and American Guidelines have suggested the high dosage of Levothyroxine for treatment begin which has been controversial [7][28]. Also, according to a systematic review, there was little evidence to represent the effects of starting dosage of Levothyroxine on the growth of newborns with congenital hypothyroidism [29]. The starting dosage of Levothyroxine was suggested as 10-15 µg/kg based on the severity of hypothyroidism [30].
The trend of 5 year-old growth of height was assessed in the current study based on the administered dosage of Levothyroxine and infants who had received lower dosage of Levothyroxine (25 µg/day>) had better trend of height growth.
The trend of weight growth of infants with congenital hypothyroidism was assessed based on the administered dosage of Levothyroxine from early infancy to 60 months of age showing a higher weight growth of infants with lower dosage of Levothyroxine (≤ 25 µg/day) as compared to the infants with higher dosage of Levothyroxine (>25 µg/day).
The newborns with congenital hypothyroidism receiving higher dosage of 9.5 µg/kg/day of Levothyroxine before 13 days of diagnosis reached to growth and development comparable to reference population in the study of Jacoba et al. [20]. Moreover, several studies reported significant growth improvement in infants with severe congenital hypothyroidism undergone administration of higher starting dosage of Levothyroxine as 10-15 µg/kg/day as well as early treatment [31]–[33]. The results of the current study showed a better physical and weight growth in infants, undergone medical treatment immediately after diagnosis (less than 28 days) as compared to the infants received treatment lately (41 days after diagnosing). Some studies demonstrated a significant correlation between the weight growth with age of treatment onset and therapeutic starting dose while a significant correlation was observed between the head circumference size with therapeutic starting dose [34]. Grant (1994) and Hashemipour et al. (2015) reported that the growth status of the infants with congenital hypothyroidism depends on the several factors such as therapeutic dose of medication [16][34].
Furthermore, a study reported the normal physical growth of the infants in first 3 years of life, immediately diagnosed and undergone treatment [35]. Nevertheless, some studies reported the latency in height growth improvement of the infants depends on LT4 dosage and, ages of treatment begin [1][36][37]. The height and weight of the patients with the age of treatment begin less than 30 days were significantly higher in some studies which are compatible with the results of the current study [34][38[39]. Moschini et al. (1986) reported the normal height growth in 6 years old with the age of treatment begin during 33 days after birth [39].
There was no significant correlation between the final height of the patients, age of treatment begin, and administered dosage of Levothyroxine in the studies carried out in Japan and Italy [14][40]. In the study conducted in Italy on a total of 215 patients during 2 decades, in spite of using screening programs and treatment, the height growth of patients was increased. However, this increase has not improved since the last 20 years and also ultimate height is not affected by the birth date, the patient’s age at the time of diagnosis, the initial dose of levothyroxine [14]. The significant effect of the starting dosage of Levothyroxine was reported on the height and weight distribution of the patients with congenital hypothyroidism in a study performed in Isfahan. The study indicated higher height and weight growth in patients received the starting dosage of Levothyroxine as ≥33.33 µg/day as compared to the subjects received lower starting dosage. Moreover, there was a significant correlation between the longitudinal growth of height and weight with the age of treatment begin and starting dosage of Levothyroxine in all percentiles [41].
The results of the current study showed the similarity of head circumference growth trend in different periods of time from early infancy to 60 months of age being statistically insignificant in the therapeutic interventions’ indices. Conversely, a study reported the significant correlation between height and weight growths with starting dose as compared to the insignificant correlation between head circumference growth with starting dose [13].
A study carried out in Isfahan showed a significant correlation between head circumference growth with starting dosage of Levothyroxine as compared to the insignificant correlation with the age of treatment begin. The head circumference growth in patients with starting dosage of Levothyroxine ≥33.33 µg/day was better in patients with starting dosage <33.33 µg/day [41]. The heterogeneity effect of normalization age of T4 was reported on head circumference as compared to the significant effect of normalization age of TSH on physical growth of patients with congenital hypothyroidism [16][34].
Conclusion
The significant effect of therapeutic interventions was reported on height, weight, and head circumference growths of patients with congenital hypothyroidism. The age of treatment begin less than 30 days plays an important role in promoting of height and weight growth of patients with congenital hypothyroidism. The better trend of height and weight growth was reported in infants with normalization time of TSH during 30 days after diagnosing. The substantial trend of weight and height growths might be demonstrated in infants with normalization age of T4 less than 14 days. The pattern of head circumference growth would be increased equally and similarly in patients after treatment.
It is suggested to refer the infants with congenital hypothyroidism to healthcare center so as to receive medical treatment immediately after diagnosing of the disease; then, the treatment must not be stopped after normalizing of serum T4 and TSH levels, the laboratory tests should be repeated afterwards, and received a significant treatment.
Suggestions
It is suggested to refer the infants with congenital hypothyroidism to healthcare center so as toreceive medical treatment immediately after diagnosing of the disease; then, the treatment mustnot be stopped after normalizing of serum T4 and TSH levels, the laboratory tests should berepeated afterwards, and received a significant treatment.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
List of abbreviations
CH: Congenital Hypothyroidism; IUGR: Intrauterine Growth Restriction; SD: Standard Deviation; TSH: Thyroid Stimulating Hormone
Ethics approval and consent to participate
The current research is the extract of MSc Epidemiology dissertation (no. 17.1.193030), Yazd University of Medical Sciences and Health Services, Iran. This study was approved by the ethical committee for MSc Epidemiology dissertation (no. 17.1.193030), Yazd University of Medical Sciences and Health Services, Iran.
Competing interests
The authors declare that there is no conflict of interest.
Funding
Yazd University of Medical Sciences, Yazd, Iran.
Authors’ contributions
All authors contributed to the design of the research. ZZ, SMR, FA, SI, SLD, MS, ID and MS collected the data. ZKH, SRP, ZZ conducted analysis and interpretation of data. All authors drafted the first version. ZKH, SRP, ZZ, SMR, KE and ID edited the first draft. All authors reviewed, commented and approved the final draft.
References
-
M
Haymond,
AM
Kappelgaard,
P
Czernichow,
BM
Biller,
K
Takano,
W
Kiess.
Early recognition of growth abnormalities permitting early intervention. Acta Paediatrica.
2013;
102
:
787-796
.
View Article Google Scholar -
G
Moradi,
Z
Khazaei,
N
Esmailnasab,
D
Roshani,
M
Zokaii,
E
Ghaderi,
B
Nouri.
The Relationship between Maternal Diseases during Pregnancy and Low Birth Weight: a Nested Case-Control Study in Rural Areas of Kurdistan Province (West of Iran). International Journal of Pediatrics.
2017;
5
:
5501-5514
.
-
MV
Rastogi,
SH
LaFranchi.
Congenital hypothyroidism. Orphanet journal of rare diseases.
2010;
5
:
17
.
View Article Google Scholar -
AJ
Wassner,
RS
Brown.
Congenital hypothyroidism: recent advances. Current Opinion in.
2015;
Endocrinology
:
Diabetes and Obesity 22
.
View Article Google Scholar -
A
Ordookhani,
P
Minniran,
R
Najafi,
M
Hedayati,
F
Azizi.
Congenital hypothyroidism in Iran. Indian journal of pediatrics.
2003;
70
:
625-628
.
View Article Google Scholar -
Y
Veisani,
K
Sayehmiri,
S
Rezaeian,
A
Delpisheh.
Congenital hypothyroidism screening program in iran; a systematic review and metaanalysis. Iranian journal of pediatrics.
2014;
24
:
665
.
PubMed Google Scholar -
A
Grüters,
F
Delange,
G
Giovannelli,
M
Klett,
P
Rochiccioli,
T
Torresani,
D
Grant,
O
Hnikova,
J
Maenpää,
GF
Rondanim.
Guidelines for neonatal screening programmes for congenital hypothyroidism. European journal of pediatrics.
1993;
152
:
974-975
.
View Article Google Scholar -
A
Ordookhani,
P
Mirmiran,
M
Hedayati,
R
Hajipour,
F
Azizi.
Screening for congenital hypothyroidism in Tehran and Damavand: an interim report on descriptive and etiologic findings, 1998-2001. Iranian Journal of Endocrinology and Metabolism.
2002;
4
:
153-160
.
-
M
Hashemipour,
M
Amini,
R
Iranpour,
GH
Sadri,
N
Javaheri,
S
Haghighi,
S
Hovsepian,
AA
Javadi,
M
Nematbakhsh,
G
Sattari.
Prevalence of congenital hypothyroidism in Isfahan, Iran: results of a survey on 20,000 neonates. Hormone Research in Paediatrics.
2004;
62
:
79-83
.
View Article Google Scholar -
E
Goodarzi,
E
Ghaderi,
S
Khazaei,
A
Alikhani,
S
Ghavi,
K
Mansori,
E
Ayubi,
B
Gholamaliee,
R
Beiranvand,
SL
Dehghani.
The Prevalence of Transient and Permanent Congenital Hypothyroidism in Infants of Kurdistan Province, Iran (2006-2014). International Journal of Pediatrics.
2017;
5
:
4309-4318
.
-
S
Kalantari,
M
Napharabadi,
F
Azizi.
The prevalence of hypothyroidism in Tehran mentally retarded patientsínstitutes. Research in Medicine.
2001;
25
:
175-178
.
-
M
Ordooei,
A
RABIE,
R
Soleimanizad,
F
Mirjalili.
Prevalence of Permanent Congenital Hypothyroidism in Children in Yazd, Central Iran. Iranian journal of public health.
2013;
42
:
1016
.
PubMed Google Scholar -
E
Mohammadi,
MR
Baneshi,
N
Nakhaee.
The Incidence of Congenital Hypothyroidism in Areas Covered by Kerman and Jiroft Universities of Medical Sciences, Iran. Journal of Health and Development.
2012;
1
:
47-55
.
-
M
Delvecchio,
MC
Vigone,
M
Wasniewska,
G
Weber,
R
Lapolla,
PP
Popolo,
GM
Tronconi,
RD
Mase,
FD
Luca,
L
Cavallo.
Final height in Italian patients with congenital hypothyroidism detected by neonatal screening: a 20-year observational study. Italian journal of pediatrics.
2015;
41
:
82
.
View Article Google Scholar -
N
Motamedi,
E
Goodarzi,
SR
Pordanjani,
R
Valizadeh,
Y
Moradi,
M
Sohrabivafa,
R
Beiranvand,
SL
Dehghani,
S
Mamdohi,
Z
Khazaei.
Incidence of phenylketonuria in Lorestan province, West of Iran (2006-2016). INTERNATIONAL JOURNAL OF PEDIATRICS- MASHHAD.
2017;
5
:
4713-4721
.
-
DB
Grant.
Growth in early treated congenital hypothyroidism. Archives of disease in childhood.
1994;
70
:
464-468
.
View Article Google Scholar -
M
Salerno,
M
Micillo,
SD
Maio,
D
Capalbo,
P
Ferri,
T
Lettiero,
A
Tenore.
Longitudinal growth, sexual maturation and final height in patients with congenital hypothyroidism detected by neonatal screening. European Journal of Endocrinology.
2001;
145
:
377-383
.
View Article Google Scholar -
P
Bain,
JE
Toublanc.
Adult height in congenital hypothyroidism: prognostic factors and the importance of compliance with treatment. Hormone Research in Paediatrics.
2002;
58
:
136-142
.
View Article Google Scholar -
KA
Selva,
A
Harper,
A
Downs,
PA
Blasco,
SH
Lafranchi.
Neurodevelopmental outcomes in congenital hypothyroidism: comparison of initial T 4 dose and time to reach target T 4 and TSH. The Journal of pediatrics.
2005;
147
:
775-780
.
View Article Google Scholar -
JJ
Bongers-Schokking,
HM
Koot,
D
Wiersma,
PH
Verkerk,
SM
de Muinck Keizer-Schrama.
Influence of timing and dose of thyroid hormone replacement on development in infants with congenital hypothyroidism. The Journal of pediatrics.
2000;
136
:
292-297
.
View Article Google Scholar -
JJ
Bongers-Schokking.
Pre-and postnatal brain development in neonates with congenital hypothyroidism. Journal of pediatric endocrinology & metabolism: JPEM (PMid:11837500).
2001;
14
:
1463-1468
.
-
M
Salerno,
R
Militerni,
C
Bravaccio,
M
Micillo,
D
Capalbo,
SD
Maio,
A
Tenore.
Effect of different starting doses of levothyroxine on growth and intellectual outcome at four years of age in congenital hypothyroidism. Thyroid.
2002;
12
:
45-52
.
View Article Google Scholar -
N
Esmailnasab,
G
Moasses,
AR
Afkhamzadeh.
Investigation of the risk factors for congenital hypothyroidism in the newborns in Kurdistan Province. Scientific Journal of Kurdistan University of Medical Sciences.
2012;
17
.
-
MH
Lotfi,
SR
Pordanjani,
M
Akhondi-Meybodi,
A
Rabei,
M
Ordooei,
B
Pourmohammadi.
Five-Year Follow Up of Physical Growth and Thyroid Hormone Levels in Infants With Congenital Hypothyroidism. Iranian Journal of Pediatrics.
2017;
27
.
-
F
Mahjoubi,
MM
Mohammadi,
M
Montazeri,
M
Aminii,
M
Hashemipour.
Mutations in the gene encoding paired box domain (PAX8) are not a frequent cause of congenital hypothyroidism (CH) in Iranian patients with thyroid dysgenesis. Arquivos Brasileiros de Endocrinologia & Metabologia.
2010;
54
:
555-559
.
View Article Google Scholar -
A
Feizi,
M
Hashemipour,
S
Hovsepian,
Z
Amirkhani,
R
Kelishadi,
M
Yazdi,
K
Heydari,
A
Sajadi,
M
Amini.
Growth and specialized growth charts of children with congenital hypothyroidism detected by neonatal screening in isfahan, iran. ISRN endocrinology 2013.
2013
.
-
MI
Surks,
E
Ortiz,
GH
Daniels,
CT
Sawin,
NF
Col,
RH
Cobin,
JA
Franklyn,
JM
Hershman,
KD
Burman,
MA
Denke.
Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. Jama.
2004;
291
:
228-238
.
View Article Google Scholar -
S
LaFranchi,
JH
Dussault,
DA
Fisher,
TP
Foley,
ML
Mitchell,
MR
Seashore,
S
Cho,
F
Desposito,
JG
Hall,
J
Sherman.
Newborn screening for congenital hypothyroidism: recommended guidelines. Pediatrics.
1993;
91
:
1203-1209
.
View Article Google Scholar -
I
Hrytsiuk,
R
Gilbert,
S
Logan,
S
Pindoria,
CG
Brook.
Starting dose of levothyroxine for the treatment of congenital hypothyroidism: a systematic review. Archives of pediatrics & adolescent medicine.
2002;
156
:
485-491
.
View Article Google Scholar -
SR
Rose,
RS
Brown,
and
Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics.
2006;
117
:
2290-2303
.
View Article Google Scholar -
JM
Dubuis,
J
Glorieux,
F
Richer,
CL
Deal,
JH
Dussault,
GV
Vliet.
Outcome of severe congenital hypothyroidism: closing the developmental gap with early high dose levothyroxine treatment. The Journal of Clinical Endocrinology & Metabolism.
1996;
81
:
222-227
.
View Article Google Scholar -
JF
Rovet,
RM
Ehrlich.
Long-term effects of L-thyroxine therapy for congenital hypothyroidism. The Journal of pediatrics.
1995;
126
:
380-386
.
View Article Google Scholar -
D
Counts,
SK
Varma.
Hypothyroidism in children. Pediatrics in review.
2009;
30
:
251
.
View Article Google Scholar -
M
Hashemipour,
Z
Heidari,
A
Feizi,
M
Amini.
Effect of Diagnostic and Treatment Factors on Growth Development of Children with Congenital Hypothyroidism: a Prospective Longitudinal Study. Iranian Journal of Endocrinology and Metabolism.
2015;
17
:
261-273
.
-
M
Delvecchio,
M
Salerno,
A
Acquafredda,
C
Zecchino,
F
Fico,
F
Manca,
MF
Faienza,
L
Cavallo.
Factors predicting final height in early treated congenital hypothyroid patients. Clinical endocrinology.
2006;
65
:
693-697
.
View Article Google Scholar -
CGD
Brook.
The effect of initial dose of thyroxine in congenital hypothyroidism on final height. Clinical endocrinology.
1997;
47
:
655-656
.
View Article Google Scholar -
DB
Grant,
I
Smith.
Survey of neonatal screening for primary hypothyroidism in England, Wales, and Northern Ireland 1982-4. Br Med J (Clin Res Ed).
1988;
296
:
1355-1358
.
View Article Google Scholar -
S
Heyerdahl,
A
Ilicki,
J
Karlberg,
BF
Kase,
A
Larsson.
Linear growth in early treated children with congenital hypothyroidism. Acta Paediatrica.
1997;
86
:
479-483
.
View Article Google Scholar -
L
Moschini,
P
Costa,
E
Marinelli,
G
Maggioni,
CM
Sorcini,
C
Fazzini,
A
Diodato,
G
Sabini,
ME
Grandolfo,
S
Carta.
Longitudinal assessment of children with congenital hypothyroidism detected by neonatal screening. Helvetica paediatrica acta.
1986;
41
:
415-424
.
PubMed Google Scholar -
M
Adachi,
Y
Asakura,
K
Tachibana.
Final height and pubertal growth in Japanese patients with congenital hypothyroidism detected by neonatal screening. Acta Paediatrica.
2003;
92
:
698-703
.
View Article Google Scholar -
Z
Heidari,
A
Feizi,
M
Hashemipour,
R
Kelishadi,
M
Amini.
Growth development in children with congenital hypothyroidism: the effect of screening and treatment variables - a comprehensive longitudinal study. Endocrine.
2016;
54
:
448-459
.
View Article Google Scholar
Comments

Downloads
Article Details
Volume & Issue : Vol 5 No 4 (2018)
Page No.: 2194-2207
Published on: 2018-04-25
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 7197 times
- Download PDF downloaded - 2168 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress