Abstract
Introduction: Atopic dermatitis (AD) is a chronic disease of the skin, involving itchy, reddish and scaly lesions. It mainly affects children and has a high prevalence in developing countries. AD may occur due to environmental or genetic factors. Currently, all therapeutic strategies involve methods to simply alleviate the symptoms, and include lotions and corticosteroids, which have adverse effects. Use of phytochemicals and natural products has not yet been exploited fully. The particle used in this study is derived from Cyamopsis tetragonoloba, an edible polysaccharide with a galactomannan component. The mannose component mainly increases its specificity towards cellular uptake by mannose receptors, highly expressed by macrophages. The aim of this study was to determine the therapeutic effect of guar gum nanoparticles (GN) in vitro and in vivo in AD.
Methods: To assess the wound healing capacity of GN, we first treated adherent fibroblast cells, with a scratch injury, with GN. GN successfully healed the wound caused by the scratch. In the in vivo experiments, Balb/c mice ears were treated topically with oxazolone (Oxa) to induce AD, and then were topically treated with GN. The ear thickness increased significantly until day 28 upon treatment with Oxa.
Results: Application of GN showed a significant decrease in ear thickness as assessed on day 28. The total cell count of skin cells that showed a fold increase, when treated with Oxa, was again decreased after topical application of GN on the affected skin. The eosinophil count, as assessed by Giemsa staining, was also increased when treated with Oxa, while GN application led to a significant decrease. Serum IgE levels were restored by GN. T helper cell and macrophage populations, when examined by flow cytometry, showed an increase in percentage when treated with Oxa; the percentage was reduced after application of GN. Hematoxylin & Eosin (H&E) staining of the ear tissue showed an increase in epidermal thickness in Oxa-treated mice, while GN application showed reduced cellular infiltration and epidermal thickness.
Conclusion: Overall, our results showed that GN, when administered topically, was successful in alleviating dermatitis caused by Oxa.
Introduction
Atopic dermatitis (AD) is a chronic condition of the skin, characterized by itchy (pruritic), reddish (erythematous) and scaly lesions [1][2]. These lesions are often localized to the flexor surfaces of the body, that is, those areas of the skin which touch when a joint bends [1]. Atopy is an inherent propensity of individuals to produce high levels of IgE in response to small amounts of environmental antigens like pollen or dust mites [2]. AD mainly affects children, and it usually sets in before the age of 2 [1][2]. Almost 75% of children with AD have remission before adolescence [2]. Often patients with AD also develop asthma or allergic rhinitis [1]. Secondary infection may develop from bacterial flora of the skin, like Staphylococcus sp. and Streptococcus sp. [1]. Diagnosis of AD is based on history and physical examination. AD can occur in three phases:
Acute atopic dermatitis: lesions are vesicular, with crust-like appearance and filled with fluid [1]. Histology of skin with acute AD shows intercellular edema and perivascular infiltration of lymphocytes [2]. There is a predominance of Th2 cytokines [3].
Subacute atopic dermatitis: lesions are dry, scaly, red and swollen [1].
Chronic atopic dermatitis: skin becomes thick and leathery in patches due to excessive scratching [1]. Histology of skin with chronic AD shows thickening of the epidermis and sparse infiltration of lymphocytes [2]. There is an increase in Th1 cytokines, such as interferon-gamma (IFN-γ) and interleukin (IL)-12 [3].
There are mainly two theories that can explain the occurrence of AD- the skin barrier hypothesis and the immunological hypothesis.
One of the main causes of AD is explained by the skin barrier hypothesis [2]. Mutations in the filaggrin gene on chromosome 1 leads to the formation of defective filaggrin protein, leading to disruptions in the epidermis, causing fissures in the skin [1][2]. The breaks in the skin allow immune cells of the dermis to come into contact with environmental antigens, causing itching, scratching and inflammation [1]. Defective filaggrin protein also leads to transepidermal water loss that causes the skin to become dry [2].
Another theory is the immunological hypothesis [2], which states that AD is caused due to an imbalance of T cells (Th1, Th2, Th17, Th22, and Treg). There is an increase in Th2 differentiation of naïve CD4+ cells in AD, leading to an increased production of IL-4, IL-5 and IL-13, and a corresponding increase in IgE levels and inhibition of Th1 differentiation.
There is no permanent cure for AD [1,4]. Current therapeutic strategies that exist only manage to alleviate the symptoms and increase the time between flare-ups [1]. Emollients or lotions can be applied regularly and liberally to reduce the dryness and itchiness [1]. The main pharmacological therapeutic strategy involves the topical application of corticosteroids [1]. Topical corticosteroids act by suppressing the release of pro-inflammatory cytokines by the immune cells, and by exerting an anti-inflammatory effect on some cells [5]. However, there are issues regarding the safety of corticosteroids, as they are associated with adverse side effects like skin atrophy and thinning of the skin [5].
Other treatments for AD include topical calcineurin inhibitors (TCIs) [1]. Calcineurin is found in the skin, where it regulates transcription factors that control early stages of T cell activation [5]. TCIs inhibit calcineurin, thereby inhibiting the activation of T cells and decreasing the release of pro-inflammatory cytokines [5,6]. TCIs do not affect healthy cells, but there have been reported cases of malignancies like skin cancer and melanomas [6]. Moreover, antibiotics may be used to treat the secondary bacterial infections, but they are not successful in treating the AD flare-ups [1]. Also, ultraviolet phototherapy and treatment with certain immunomodulatory agents have been helpful for patients who do not respond to conventional therapies [1].
Due to the adverse effects of the current therapies, there is great interest to develop treatments (drugs) that can better control the symptoms of AD with minimal side effects [4]. Many new drugs inhibit different signaling molecules in key pathways. Omalizumab is a murine monoclonal antibody against serum IgE, which increases significantly in AD [4]. Dupilumab is a human monoclonal antibody directed against the α subunit of the IL-4 receptor (IL-4Rα), which binds 3 both IL-4 and IL-13 [4][5]. Lower expression of these cytokines leads to increase in skin barrier proteins like filaggrin. Additionally, crisaborole is a non- steroidal phosphodiesterase IV (PDE IV) inhibitor [4][5]. PDE4 inhibition leads to accumulation of cAMP and the subsequent inhibition of pro-inflammatory cytokines [4][5].
Not much has been established about the effects of alternative therapies [1], and the potential of natural substances in the treatment of AD has not yet been fully explored [3]. Some plant products have been found to be effective in alleviating the symptoms of AD. Extracts of a variety of potato (Solanum tuberosumL. cv Hongyoung), containing anthocyanins, have been found to impart health-protective effects in various diseases, including AD, due to their antioxidant and anti-inflammatory activities [3]. Extracts of cacao, the main component of chocolate, is rich in polyphenols, especially flavonoids [7]. It has been found effective in reducing the symptoms of AD, probably by altering the balance between the Th1 and Th2 cytokines [7]. Quercetin, a plant flavonoid, can alleviate AD by inhibiting pro-inflammatory cytokines [8]. Application of an extract of Lentinula edodes (shiitake mushrooms) to mice with AD-like lesions led to marked reduction in symptoms, including reduction in lesions, serum IgE, mast cell infiltration, and Th1 and Th2 cytokines (in the ear) [9].
Neutrophils are key players in inflammation. They have been found to be involved in different phases of inflammatory skin diseases, such as AD [1][10]. Treatment with anti-GR1 antibody has been found to be effective in reducing the common symptoms of AD, like ear swelling and T cell infiltration [1]. Patients with AD have higher neutrophil counts, as well as higher neutrophil-to- lymphocyte ratios, which serve as a marker to assess the severity of the disease [10].
With the harmful effects of topical corticosteroids in mind, we designed a study where we used a compound derived from natural sources, which would not be as harmful as steroids. We used guar gum nanoparticle, topically, to treat AD. Guar gum (GG) is a high molecular weight, non- ionic polysaccharide gum present in the seeds of the leguminous plant Cyamopsis tetragonoloba [11]. It has a viscous, gel-like consistency in water, and is a galactomannan, containing galactose and mannose, usually in a 1:2 ratio [11]–[13]. It has antimicrobial and antiproliferative effects [12]. The guar gum has been modified in such a way as to facilitate uptake by cells [14]. This modified guar gum nanoparticle (GN) is a spherical molecule with a size of about 80 nm [14].
The main objective of this study is to check the wound healing effect of GN in vitro and in vivo in mice model. The in vitro study on scratch wound assay was done in NIH3T3 cells where the scratch was introduced in a monolayer culture of adherent NIH3T3 cells. The migration of cells over the induced scratch is indicative of wound healing in vitro. The comparative analysis of the width in the absence and presence of GN will demonstrate whether GN shows any wound healing property. The in vivo model of AD addresses the therapeutic effect of GN on skin lesions formed during AD. Induction of AD was achieved by Oxa administration [15]; GN administration should heal those lesions, reducing the redness and swelling of the skin, as hypothesized in this study. Varied assays, such as total cell count, clonogenic potential, serum IgE, flow cytometry and histological analysis, indicated the efficacy of GN in ameliorating the disease.
Methods
Guar gum nanoparticle (GN)
2 mg/ml guar gum nanoparticle (GN) stock was obtained from the laboratory of Dr. Arup Mukherjee, Department of Chemical Technology, University of Calcutta, Kolkata, India, where it was prepared by using protocols previously published by Ghosh SK et al. [16].
Cell line
The NIH3T3 fibroblast cells were obtained from the National Centre for Cell Science, Pune. The cells were maintained and cultured in our laboratory using DMEM (Himedia, Inc., India), supplemented with 10% FBS (Himedia, Inc.) and 1% Penicillin-Streptomycin (Himedia, Inc.).
In Vitro Scratch Assay
Upon reaching 80% confluency, NIH3T3 fibroblast cells were plated into 6-well tissue culture plates (Tarsons, Inc., India) in 3 groups:
Group I - Only scratch
Group II - Scratch, followed by treatment with 40 µg/ml GN
Group III - Scratch, followed by treatment with 100 µg/ml GN.
The cells were allowed to adhere at 37°C in a 5% CO2 humidified incubator (Thermo Fisher, Waltham, MA). After the cells adhered, a scratch was made in the wells of Group I, Group II and Group III. A 2-200 µl microtip was used to make a single scratch along the middle of the well. After making the scratch, the medium was discarded and the cells were washed with 1X PBS. Fresh medium was added; then 40 µg/ml GN was added to the well of Group II, and 100 µg/ml GN was added to the well of Group III. Migration of the cells over the scratch was observed at a magnification of 20X using an inverted light microscope (Nikon TiS) over a period of 24 hours. The width of the gap was measured, and the percentage decrease in the gap width over time was calculated and plotted graphically.
Ethical approval
All experiments were performed according to rules determined by the Institutional and Departmental Animal Ethics Committee. Animals were housed under specific pathogen-free conditions at the animal house of the Department of Zoology, University of Calcutta.
Mice
6-8 week old Balb/c mice were purchased from the National Institute of Nutrition, Hyderabad, India. They were housed in the animal house facility of the Department of Zoology, University of Calcutta. They were maintained under specific pathogen-free conditions with 12 hrs light and dark cycle. Six-week old mice were taken and divided into three experimental groups (n=3):
Group I - Control- mice were free from any treatment
Group II - Oxa- mice were treated with Oxazolone
Group III - GN- mice were initially treated with Oxazolone, and then with GN.
Induction of disease
The ears of the mice in the Oxazolone group were topically sensitized with 40 µl of 0.8% Oxazolone (Sigma-Aldrich, St. Louis, MO), dissolved in acetone (SRL, India) on day 0, and challenged with 0.4% Oxa on days 7, 10, 12, 14, 17, 19, 21, 23, 25 and 27. The topical administration was done using a microtip attached with a micropipette (Tarsons, Inc.).
Treatment with GN
Mice were treated with GN on days 14, 17, 19, 21, 23, 25 and 27. Then, 50 µl of 2 µg/ml GN (100
µg GN) was administered topically on each ear of the mice of Group III.
Assessment of ear thickness
The thickness of the ears of the mice in all the three groups were measured on days 0, 7, 10, 17, 19, 21, 23, 24, 26 and 27, using a digital slide caliper (Mitutoyo South Asia Pvt. Ltd.).
Collection of samples
All the animals from each group were sacrificed on day 28 by cervical dislocation, and peripheral blood and ear tissues of mice were collected.
Peripheral blood
Peripheral blood was collected by cardiac puncture in K2-EDTA blood collection tubes (BD, Franklin Lakes, NJ).
Ear tissue: Ear tissue for histology was collected in 10% buffered formalin. For other assays, ear tissue was collected in DMEM. After collecting ear tissues in DMEM, they were cut into small pieces and incubated in DMEM, containing 5 mg/ml Dispase (Gibco) and 1 mg/ml Collagenase IV (Himedia, Inc.), for at least 2 hours at 37°C with shaking. The digested cell suspension was passed through a mesh and the cells were collected.
Cell count
Peripheral blood
Peripheral blood was mixed with an equal volume of Trypan blue dye (Himedia, Inc.) and then cell viability was determined using a hemocytometer.
Ear tissue
The cell suspension of ear tissue was mixed with an equal volume of Trypan blue dye (Himedia, Inc.), and the number of live cells were counted using a hemocytometer.
Differential cell count of peripheral blood
A drop of blood was placed on a microscope slide and, using another slide, a smear was prepared. The blood smears were air dried, fixed with methanol (SRL, India), and stained with Giemsa (Merck) for 15 minutes (Ramnik Sood, 2009). They were repeatedly washed with tap water to remove excess stain and observed under a microscope (Dewinter Fluorex LED) at 40X magnification. The cell types were distinguished on the basis of their nuclear morphology.
CFU-c assay for clonogenic potential
CFU-c assay was done using methyl cellulose semi- solid medium. The CFU-c medium was prepared using IMDM (Himedia, Inc.), supplemented with 30% FBS (Himedia, Inc.), 1% penicillin-streptomycin (Himedia, Inc.), 10% BSA (Biosera), and 5 ng/ml murine SCF (Biovision). Finally, 1.5% methyl cellulose (Himedia, Inc.) was added and mixed well. One ml of the medium was mixed with 1 ml cell suspension and plated in wells of a 24-well plate (Nest Biotech), and incubated at 37°C with 5% CO2 in a humidified incubator (Thermo Fisher). The number of colonies formed was counted after 6 days. The clonogenic potential was calculated by dividing the total number of colonies observed in all the fields by the number of cells plated.
Serum IgE estimation
Levels of IgE in the serum were estimated by direct ELISA, using a protocol modified from that provided by BD (BD OptEIA Mouse IgE ELISA Set). The wells of a high-binding, 96-well ELISA plate (Corning, USA) were coated with serum samples, and diluted 1:20 in blocking buffer (1% BSA in PBST), then left overnight at 4°C. The wells were washed well with PBS-Tween solution (PBST), and then 1 µg/ml rat anti-mouse IgE-biotin (BD) in blocking buffer was added for 1 hour at room temperature. After washing the wells thoroughly with PBST, 1:1000 Avidin-HRP (BD) was added to the wells for 30 minutes at room temperature, washed, and developed using TMB substrate (Sigma-Aldrich). The absorbance was measured at 405 nm using a multi-well plate reader (Thermo Fisher). The concentration of IgE in the serum samples was calculated using a standard curve prepared with purified IgE (BD).
Antibody staining solutions for flow cytometry
Flow cytometry was done using the following sets of antibodies: 1:50 anti-mouse CD 45- PerCP/Cy5.5 (BioLegend) + 1:50 anti-mouse CD3-PE (BD) + 1:50 anti-mouse B220-FITC (BD) in 1X PBS.1:50 anti-mouse CD3-PE (BD) + 1:50 anti-mouse CD4-V450 (BD) + 1:50 anti-mouse CD8-AlexaFluor488 (BD) in 1X PBS.1:50 anti-mouse CD 45-PerCP/Cy5.5 (BioLegend) + 1:50 anti-mouse GR1-FITC (MACS Technology) + 1:50 anti-mouse F4/80-PE (Invitrogen) in 1X PBS.
Flow cytometry
Peripheral blood
Fifty µl of the blood collected in the K2-EDTA tubes was taken in a 15 ml centrifuge tube (Tarsons, Inc.). To it, 50 µl of the staining solution (containing a mixture of antibodies) was added and incubated for 15 minutes at room temperature. The 10X RBC lysis buffer (Himedia, Inc.) was diluted to 1X using distilled water; two ml of 1X RBC lysis buffer was added to the antibody- stained blood for 15 minutes at room temperature and centrifuged at 1500 rpm for 5 minutes at room temperature. The supernatant was decanted and the pellet washed with FACS buffer (5% FBS and 0.3% sodium azide in 1X PBS), by centrifuging at 1500 rpm for 5 minutes at room temperature. The supernatant was pipetted out and the pellet resuspended in PBS. Flow cytometry was done on the stained cells using BD FACSAria (BD Biosciences, San Jose, CA, USA), and the data were analyzed using the BD FACSDiva software (BD Biosciences).
Ear Tissue
After digestion, the cell suspension obtained from the ear tissue was strained, washed with FACS buffer (centrifuged at 4000 rpm, at 4°C for 5 minutes), and the pellet resuspended in PBS. The cells were incubated with the staining solution for 15 minutes at room temperature. The stained cells were then centrifuged at 4000 rpm, at 4°C for 5 minutes, and the pellet was resuspended in 1X PBS. Flow cytometry was done with the stained cells using BD FACSAria, and the data were analyzed using the BD FACSDiva software. The cells obtained from the ear tissue were pooled from three mice from each group.
Histology
Tissue sectioning
The ear tissues were fixed in 10% buffered formalin (in 1X PBS). Tissue processing was done using 70% ethanol, 100% ethanol (Merck), xylene (SRL) and paraffin (Himedia, Inc.). The tissues were embedded in paraffin; 5 µm tissue sections were sliced using a microtome. The tissue sections were further stretched in a water-bath maintained at 40°C, and collected onto the slides coated with Mayer’s albumin.
Hematoxylin-Eosin (H&E) staining
The tissue sections were de-paraffinized using xylene and hydrated using ethanol in decreasing concentrations (100%, 90%, and 70%), stained with Hematoxylin Eosin, and again dehydrated using increasing concentrations of ethanol (70%, 90%, and 100%) and xylene. The slides were mounted using DPX mountant (Merck) and observed at 40X magnification under a light microscope (Dewinter Fluorex LED).
Statistical analysis
All data are presented as Mean ± SEM. Statistical significance was calculated using t-test; all values of p0.05 were considered as significant. GraphPad Prism 6 (GraphPad Software, Inc, San Diego, CA, USA) was used for statistical analysis.
Results
In vitro Scratch Assay
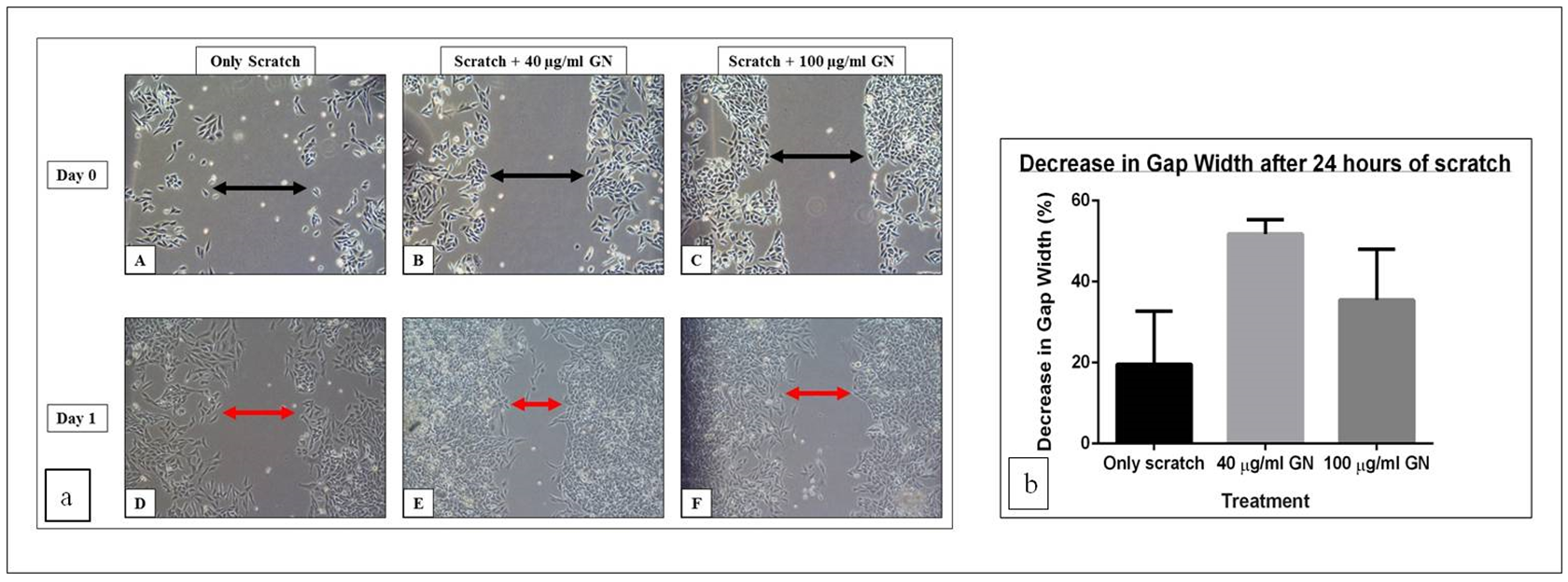
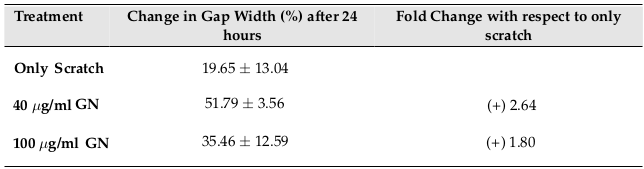
Fibroblasts are involved in the normal wound healing processes by creating an extracellular matrix and collagenous structures to support the growth of other cells, as well as by contr acting the wound [17]. Since fibroblasts already have an inherent healing property, the width of the scratch in the untreated cells also decreased, even without treatment. However, treatment with GN sped up the process of healing, and the width of the scratch reduced more in the same amount of time. The width of the gap created by the scratch was measured on day 0 (immediately after treatment) and on day 1 (24 hours after treatment). There was a 19.65% decrease in the gap width after 24 hours in the well containing scratch only ( Figure 1a (A,D)), indicating that the cells, by themselves, had attempted to close the gap, thereby healing the wound. On the other hand, after treatment with 40 µg/ml GN, the gap width decreased 51.79% ( Figure 1a (B,E)), while treatment with 100 µg/ml GN reduced the gap width by 35.46% ( Figure 1a (C,F)). Thus, 40 µg/ml GN is 2.64- fold more, and 100 µg/ml GN is 1.80-fold more, effective in reducing the gap width compared to untreated cells. Also, 40 µg/ml GN is 1.46-fold more effective than 100 µg/ml GN in reducing the gap width ( Figure 1b ). This observation confirmed that GN, at a concentration of about 40 µg/ml, could be used in in vivo wound healing models.
GN treatment reduced the clinical symptoms of AD
Mice treated with Oxa showed marked symptoms of the disease. By day 7 ( Figure 2B ), the ear and the surrounding skin started to turn red and swell. By day 14 ( Figure 2C ), the ear became red and appeared dry. The fur around the ear started to shed. By day 28 ( Figure 2E ), a large area of the fur had shed and the whole area, including the ear, was dry and swollen. On the other hand, topical treatment with GN seemed to have had a marked therapeutic effect on the disease. Figure 2F shows that the skin near the ear had healed and was not as red or swollen as the Oxa- treated mice on the same day.
GN treatment reduced swelling of the ears
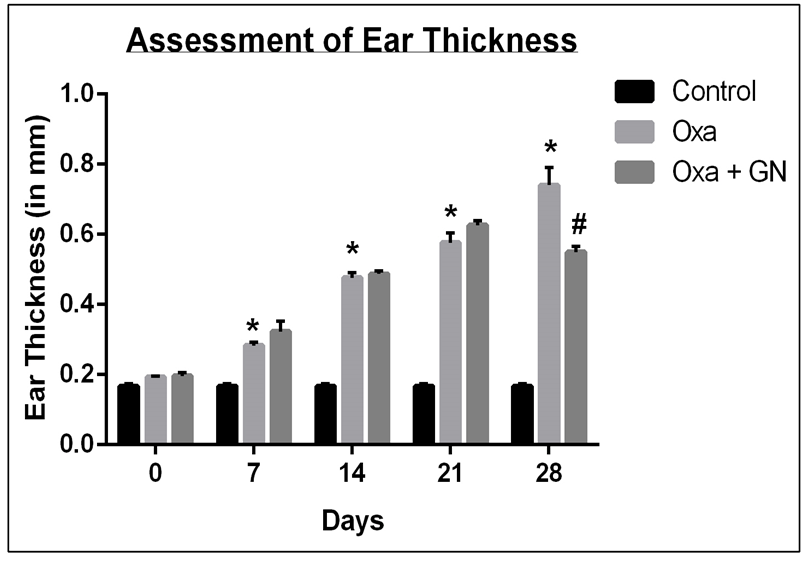
Due to treatment with Oxa, the ears had swollen and the thickness of the ear increased significantly. Treatment with GN reduced the thickness, and thus, the swelling, significantly by day 28. Oxa-treatment led to a progressive increase in the ear thickness- 2.82-fold increase (p0.05) on day 14, 3.41-fold increase (p0.05) on day 21, and 4.35-fold increase (p0.05) on day
28. Treatment with GN was started after 14 days of Oxa treatment, once the swelling and redness was significant. The thickness initially increased up to day 21, after which it started to decrease. On day 28, there was a significant, 1.35-fold decrease (p0.05) as compared to Oxa ( Figure 3 ).
GN reduced infiltration of cells
As is common in any inflammatory condition, Oxa treatment led to an influx of immune cells to the site of inflammation, that is, the ear, via the blood. GN treatment successfully inhibited this infiltration.
Peripheral blood
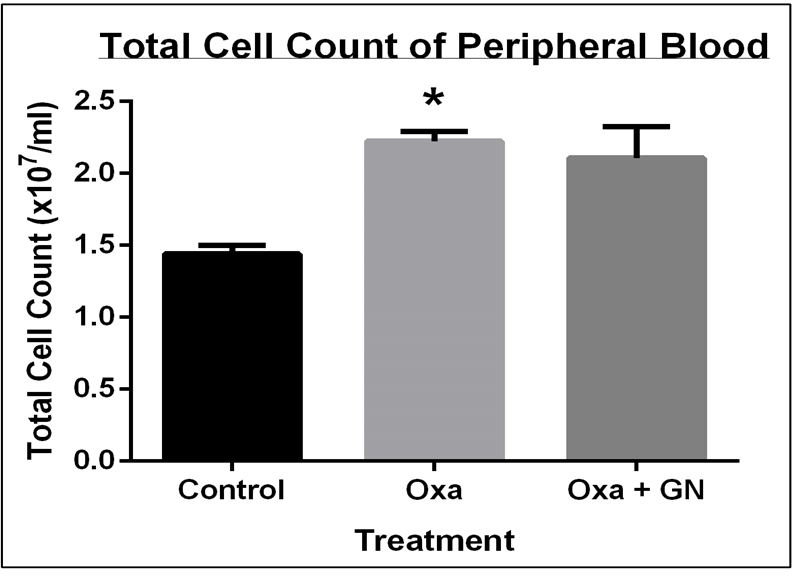
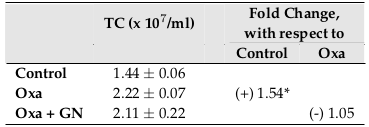
Treatment with Oxa led to a 1.54-fold (p0.05) increase in the total cell count of the blood compared to the untreated control, indicating that more cells are being transported by the blood to combat the inflammation. Topical treatment with GN led to a slight decrease (1.05-fold) in the total cell count of blood, showing its ability, though not significant, to inhibit the infiltration ( Figure 4 ).
Ear tissue
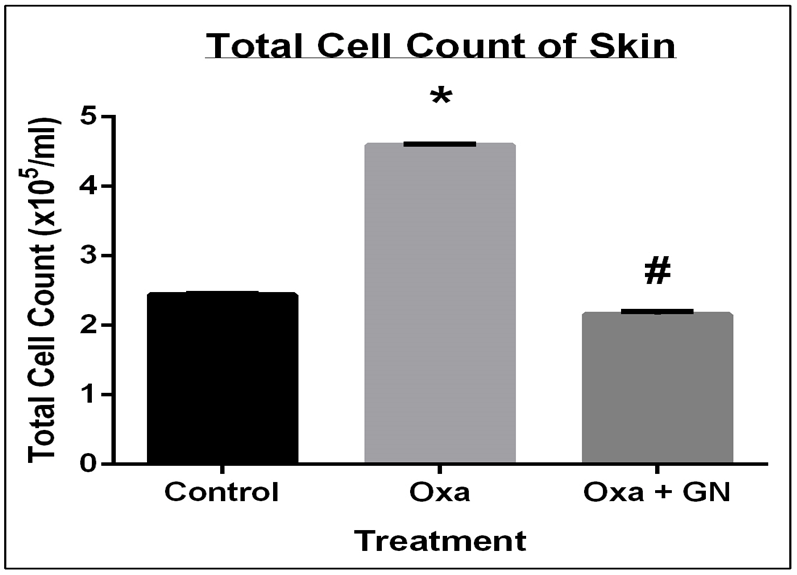
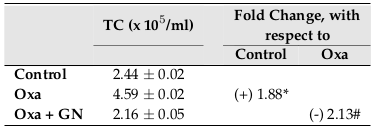
The total cell count of the site of inflammation (i.e., the skin) increased 1.88-fold (p0.05) after 28 days of treatment with Oxa, indicating the presence of inflammation. GN significantly reduced the cell count by 2.13-fold (p0.05), indicating that it is significantly successful in reducing inflammation when applied topically ( Figure 5 ).
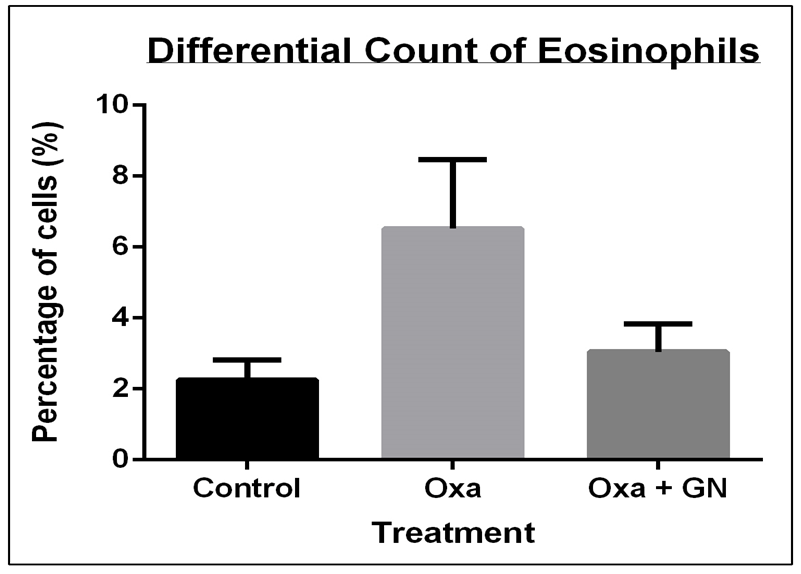
GN reduced the eosinophil count in the blood
In AD, there is an increase in eosinophils and a corresponding eosinophilia in the blood [10][18][19]. Differential count of the eosinophils in the blood showed a 2.88-fold increase with Oxa treatment, which was reduced with GN treatment by 2.14-fold ( Figure 6 ).
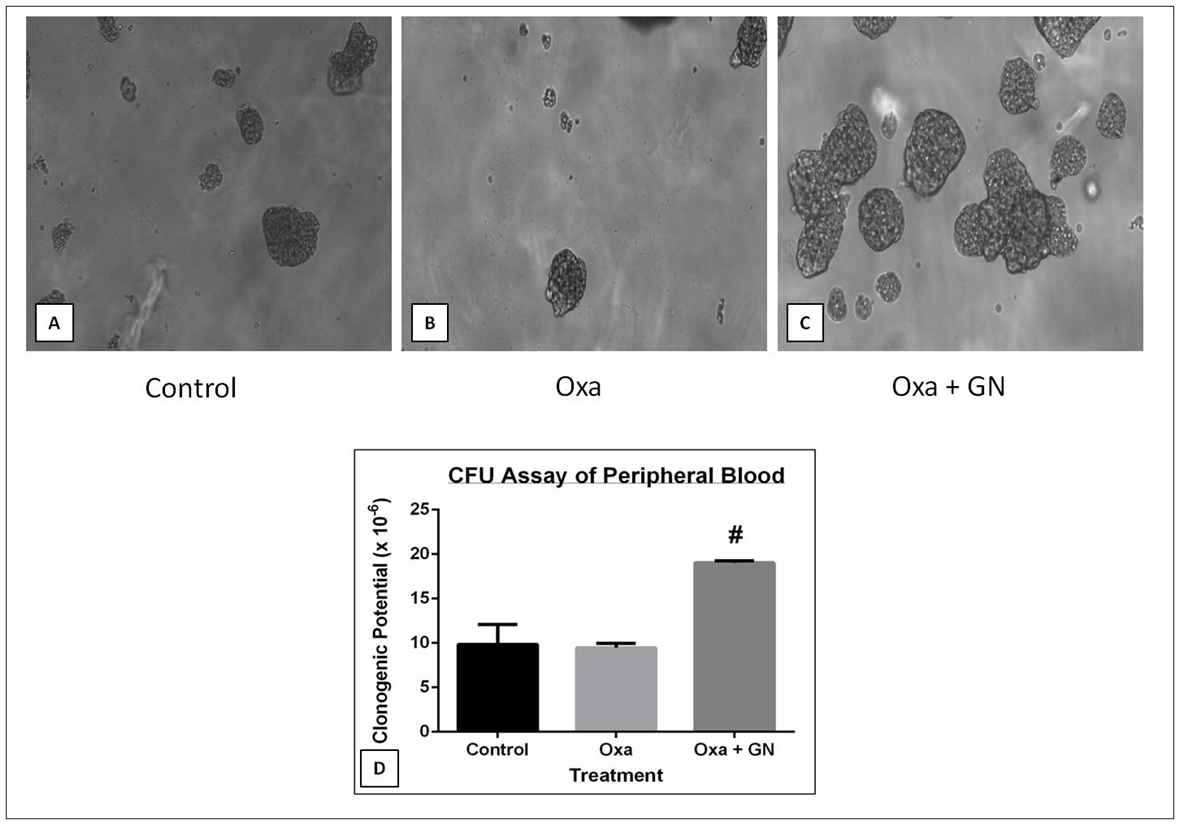
GN successfully restored clonogenic potential
Clonogenic potential is a measure of the ability of cells to form colonies. Cells under stress conditions generally lose some of their clonogenic potential.
Peripheral blood
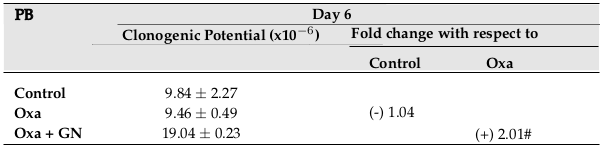
After 6 days of incubation, there was a 1.04-fold decrease in clonogenic potential of blood from mice treated with Oxa, as compared to control. Treatment with GN restored the clonogenic potential, which increased 2.01-fold (p0.05) compared to Oxa ( Figure 7 ).
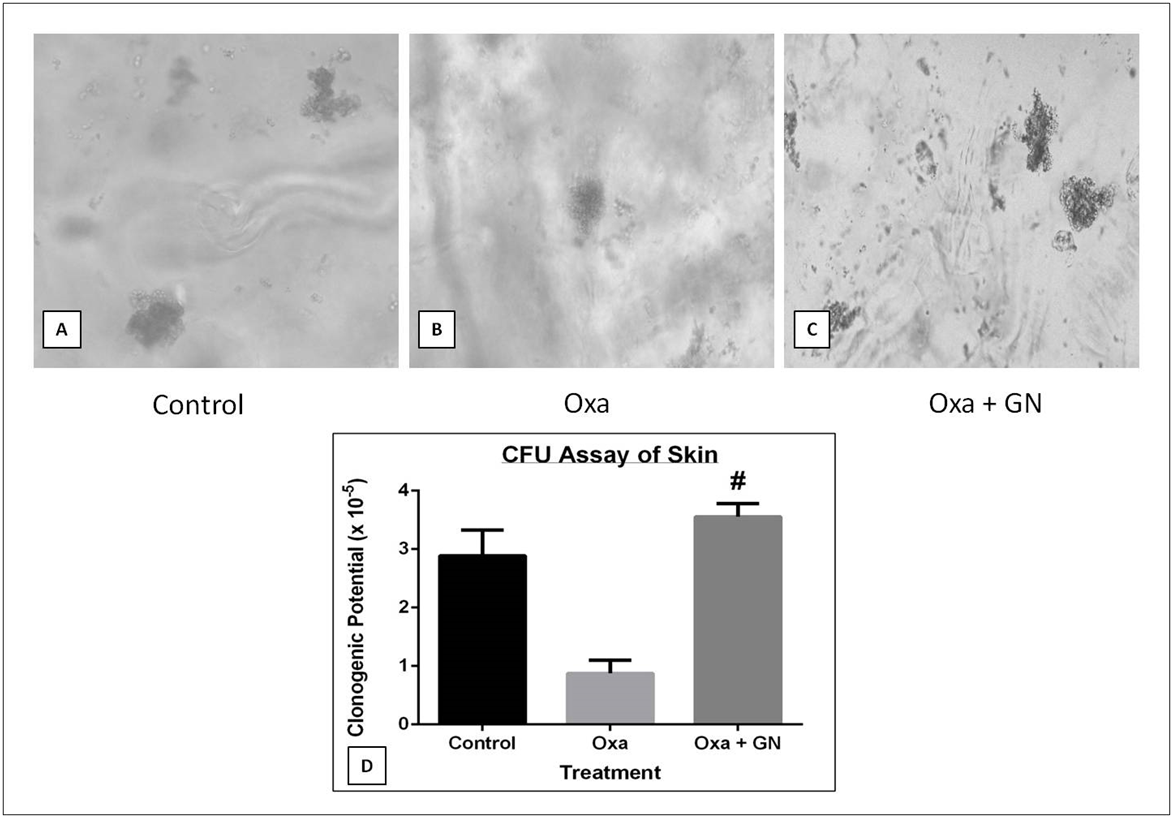
Ear tissue
After 6 days, there was a 3.28-fold decrease in the clonogenic potential of skin cells of the mice treated with Oxa, compared to control. Treatment with GN led to a 4.05-fold (p0.05) increase in the clonogenic potential compared to Oxa ( Figure 8 ).
GN successfully reduced serum IgE levels
In AD, there is an increase in the level of IgE in the serum [2][4]. With Oxa treatment, the level of IgE in the serum increased by 3.43-fold (p0.05), as compared to the control. GN treatment led to a 1.83-fold (p0.05) decrease in the serum IgE, as compared to Oxa ( Figure 9 ).
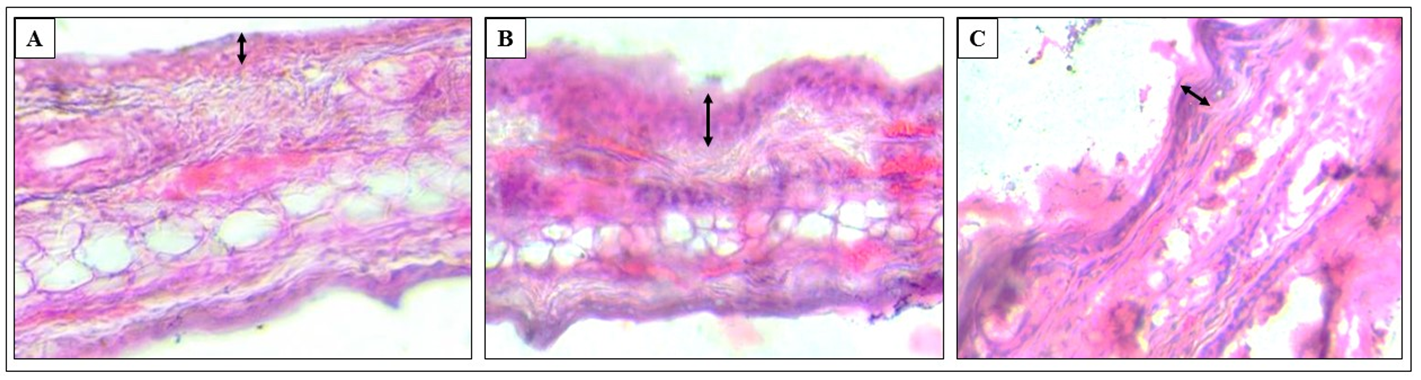
Histology
Due to AD, the epidermis thickens [4], and eosinophils and mast cells in the skin increaseliuu [19], as seen by HE staining ( Figure 10 ).
GN inhibited differentiation of CD4+T cells and neutrophils
According to previous studies, there is an increased Th2 differentiation of CD4+T cells under conditions of AD [4]. Potential therapeutic agents aim to restore the balance of T cells, by inhibiting the differentiation of CD4+cells, which in turn reduces the expression of pro- inflammatory cytokines. Also, the neutrophil count was found to increase significantly in patients with AD [10][20]. A study showed that treatment with anti-GR1 antibody was successful in preventing ear swelling and infiltration of T cells in a mouse model of AD [20]. Our observations with both peripheral blood and ear tissue confirmed the involvement of CD4+T cells and neutrophils in AD since, in both tissues, there was a significant increase in these populations.
Peripheral blood
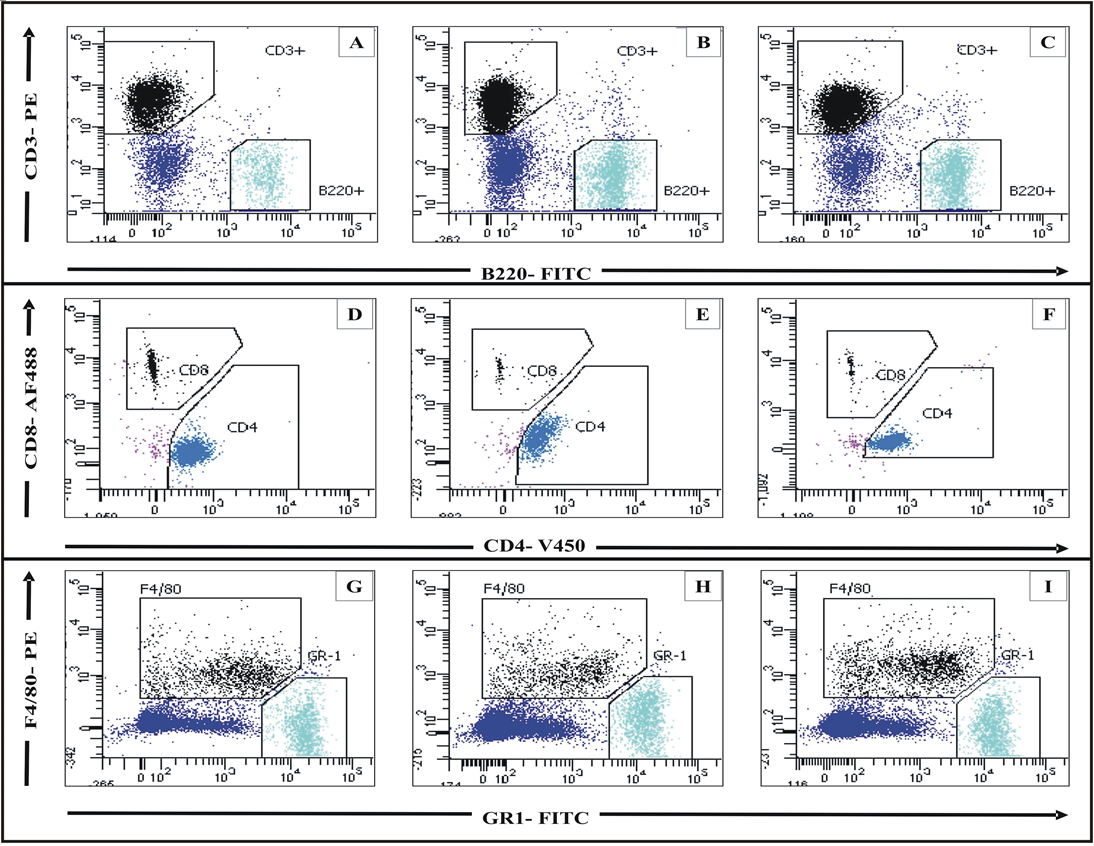
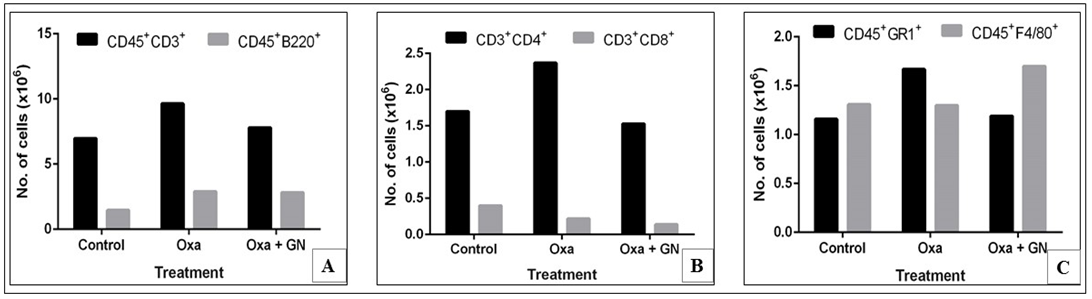
Flow cytometry ( Figure 11 and Figure 12 ) of CD45+cells of the peripheral blood of mice treated with Oxa, showed a 1.38-fold increase in the CD3+T cell population. There was not much change in the population of CD45+B220+cells. Among the CD45+CD3+cells, there was a 1.40- fold increase in the CD4+population, compared to control. There was also a 1.44-fold increase in the CD45+GR1+neutrophil population. After treatment with GN, there was a 1.23-fold decrease in the CD45+CD3+cells and a 1.55-fold decrease in the CD3+CD4+population. Also, the CD45+GR1+neutrophil population decreased by 1.40-fold.
Ear tissue
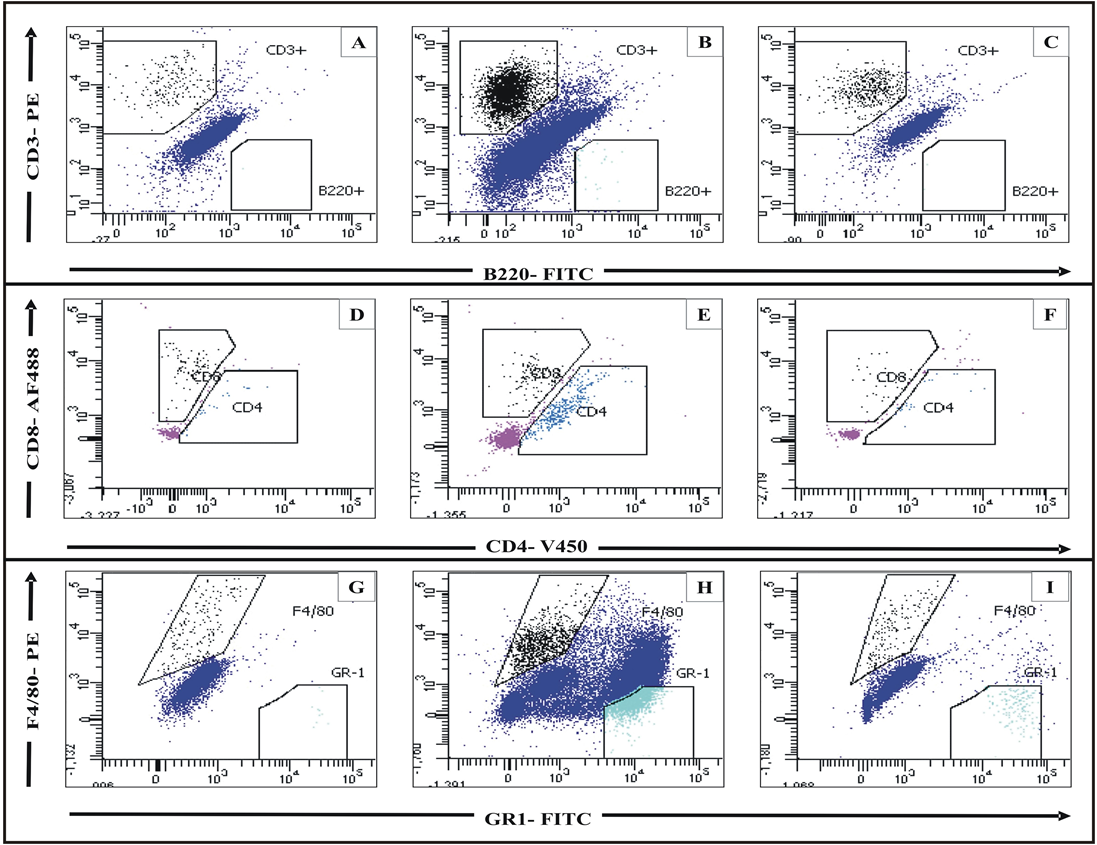
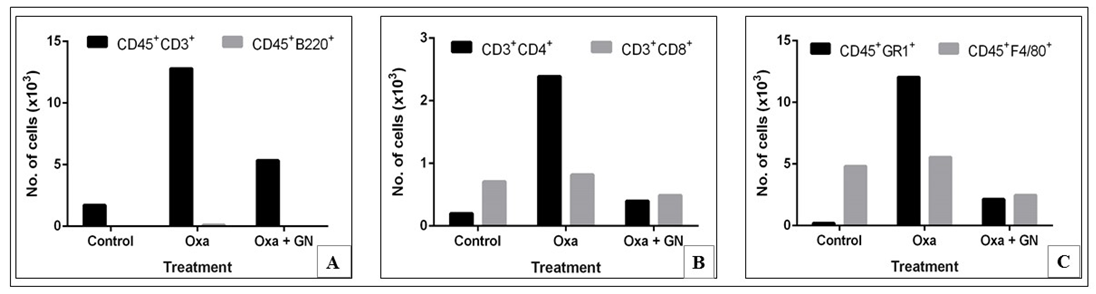
Flow cytometry ( Figure 13 and Figure 14 ) of CD45+ cells of the ear tissue, of mice treated with Oxa, showed a 7.44-fold increase in the CD3+T cell population. Among these CD45+CD3+ cells, there was an 11.68-fold increase in the CD4+ population, compared to control. While there was only about 0.21 x 103CD45+GR1+neutrophils in the control, Oxa treatment led to a massive infiltration (12.05 x 103neutrophils), leading to a massive 56.22-fold increase. After treatment with GN, there was a 2.39-fold decrease in the CD45+CD3+cells and a 5.99-fold decrease in the CD3+CD4+population. Also, the CD45+GR1+ neutrophil population decreased by 5.62-fold. Ear tissue hardly showed the presence of CD45+B220+ cells. Both the CD3+CD8+ and the CD45+F4/80+ populations decreased with GN treatment.
Discussion
Atopic dermatitis is quite a common disease, with a prevalence of about 20% in developing countries [21]. To date, therapy for AD includes steroids and certain inhibitors, which are known to have adverse effects. To overcome this problem, we attempted to use a nanoparticle made from guar gum, which is a natural product, and is known for its anti-proliferative effects [12]. We postulated that guar gum nanoparticle, or GN, may have fewer adverse effects than steroids. It is also easily available since guar is cultivated in many parts of India [22].
Our previous in vitro studies have shown that GN is taken up by all types of cells, be it cell
lines like RAW 264.7 macrophages and NIH 3T3 fibroblasts, or primary cultures. GN is especially taken up by cells expressing mannose receptors on their surface due to its mannose-rich nature. We have also found that GN has anti-inflammatory activity in in vitro inflammatory models, as well as in a mouse model of peritonitis. This study has been done to assess whether the GN has any therapeutic effect in atopic dermatitis.
In AD, the symptoms include lesions on the skin, which are the result of disruption in the epidermis. In vitro scratch assay has been done to determine whether GN has any wound healing capability. The in vivo experiment was done using a mouse model of atopic dermatitis, induced using oxazolone, where the anti-inflammatory activity of GN was assessed in the disease. In our study, we found that GN was successful in healing the wound-induced in the monolayer of fibroblasts. We also found that it successfully reduced the symptoms of AD, when applied topically.
GN, at a concentration of 40µg/ml, was able to heal the scratch wound, as assessed by the decrease in the gap created by a scratch, by 2.64-fold, compared to untreated cells. While the untreated cells were able to decrease the gap by approximately 20%, the cells treated with 40
µg/ml GN reduced the gap by over 50%, in the same duration of time ( Table 1 ; Figure 1ab ).
This indicates that although the untreated fibroblasts themselves also close the gap using their inherent healing properties [17], GN successfully speeds up the process of healing.
Visible symptoms of AD include redness, swelling, dry skin, and rashes. After 28 days of treatment with oxazolone, the mice suffered severe redness and dryness of the skin, along with a tremendous loss of fur in the area where Oxa was administered ( Figure 2E ). A 14-day treatment with GN, following 14 days of Oxa, significantly reduced the swelling. There was much less loss of fur as well ( Figure 2F ). These symptoms were correlated to the thickness of the ear. With Oxa treatment, the thickness of the ear increased progressively over 28 days. While the thickness on day 0 was 0.19 mm, after 28 days, it increased to 0.74 mm, showing a 4.35-fold (p0.05) increase. On the other hand, the ear thickness of mice treated with GN showed an increase until day 21 (from 0.20 mm on day 0 to 0.63 mm on day 21), and then a decrease to 0.55 mm (1.35-fold decrease, p0.05) on day 28. This indicates that GN does, indeed, reduce the swelling caused by AD, but it takes some time for the action ( Table 2 ; Figure 3 ).
Inflammation leads to an infiltration of immune cells to the site of inflammation. These immune cells are generally transported to the site by the blood, as a result of which the number of cells in the blood also usually increase. Cellular infiltration does increase, both in the blood and in the ear tissue, with Oxa treatment (1.54-fold increase and 1.88-fold increase for blood and skin, respectively; p0.05), which is reduced with GN treatment ( Table 3 ; Table 4 ; Figure 4 and Figure 5 ). GN appears more successful in reducing the infiltration in the skin, as compared to the blood, with a 2.13-fold (p0.05) decrease. In AD, there is an increase in the population of eosinophils in the blood [10][18][19]. GN successfully reduced the influx of eosinophils in the blood that had increased after treatment with Oxa ( Table 5 ; Figure 6 ).
Clonogenic potential is a measure of a population’s ability to proliferate and grow into colonies. Oxa treatment led to a loss of clonogenic potential, in both the blood and skin. GN restored the clonogenic potential of both the tissues significantly ( Table 6 and Table 7 ; Figure 7 and Figure 8 ). Serum IgE, which increased with Oxa, also decreased with GN ( Table 8 ; Figure 9 ). Histological sections show thickening of the epidermis of Oxa- treated mice ( Figure 10 ). The epidermis is quite thin after GN treatment.
Our initial studies with GN on AD showed that it alleviated the symptoms. However, the exact mechanism was not known. Flow cytometry was done to assess the effect of GN on the different cellular subsets. It is hypothesized that AD is caused by an imbalance in the populations of T cells [2]. There is an increase in the population of CD4+T cells, and also in the population of GR1+ neutrophils [10][20]. These facts are supported by our observations. With Oxa treatment, the populations of CD45+CD3+T cells, CD3+CD4+Th cells and CD45+GR1+ neutrophils increased in the blood. These populations decreased with GN treatment ( Figure 11 and Figure 12 ). Neither Oxa nor GN had much effect on the CD45+B220+B cells, CD3+CD8+Tc cells, or the CD45+F4/80+ macrophages. In the skin, as well, with Oxa treatment, the populations of CD45+CD3+T cells, CD3+CD4+Th cells, and CD45+GR1+neutrophils also increased. These populations decreased with GN treatment ( Figure 13 and Figure 14 ). The population of CD45+B220+B cells in the ear was negligible. There was a slight increase in the CD3+CD8+Tc cells and the CD45+F4/80+macrophages, with Oxa treatment, which reduced slightly with GN, but these changes were not significant.
Taken together, these observations indicate that GN may play a role in restoring the balance between Th1 and Th2 cytokines, like some therapeutics [7][9]. Further studies are ongoing to determine whether GN inhibits pro-inflammatory cytokines like quercetin does [8], or whether GN somehow acts on the filaggrin protein and removes the disruptions to the epidermis.
To the best of our knowledge, this is the first study to evaluate the anti-inflammatory and wound healing properties of GN. However, further research on the signaling pathways and associated cytokines are yet to be done; these will help elucidate the exact mechanism(s) of action of GN in treating (alleviating) AD.
Conclusion
As there is no permanent cure for atopic dermatitis, the use of nanoparticles derived from endosperm of guar gum seeds has been explored in this study. The in vitro study showed successful wound healing effect of GN in the scratch wound assay using NIH3T3 cells. The in vivo study showed that GN successfully decreased the symptoms of AD (e.g. redness of the skin and epidermal thickness) as well decreased serum IgE levels, and total counts for blood cells, skin cells, eosinophils, macrophages and neutrophils. GN restored the decreased clonogenic potential in the presence of Oxa. From our study, it can be concluded that GN can be used as an anti- inflammatory, anti-allergic and pro-regenerative agent. However, signaling based studies should be conducted to obtain insight into its mechanism of action.
List of abbreviations
AD: Atopic dermatitis; BSA: Bovine Serum Albumin; CD: Cluster of differentiation; DMEM: Dulbecco’s Modified Eagle’s Medium; FBS: Fetal Bovine Serum; GN: Guar gum nanoparticle; IFN-γ: Interferon-γ; IgE: Immunoglobulin E; IL: Interleukin; IMDM: Iscoves’ Modified Dulbecco’s Medium; K2-EDTA: Potassium salt of ethylene diamine tetra acetic acid; Oxa: Oxazolone; PBS: Phosphate Buffered Saline; PBST: PBS + Tween 20; RBC: Red blood cell; SCF: Stem Cell Factor; SEM: Standard error of mean; Th cells: T helper cells; TMB: 3,3’,5,5’- Tetramethylbenzidine
Competing interests
The authors have declared that no conflict of interest exists.
Funding
The work has been funded from the corresponding author’s grant with the Indian Council for Medical Research (ICMR), New Delhi. We also wish to acknowledge UGC-SAP, UPE-Phase II and DST-FIST for providing departmental infrastructure.
Availability of data and materials
Supplementary data shall be made available if asked for.
Authors’ contributions
NG SM have contributed equally in performing the experiments, analyzing the data and writing the manuscript. ERB initiated the project with her idea, designed the experiments, analyzed all data and wrote the manuscript.
References
-
JAGW
Blajer,
K
Mariusz.
The Inverse Simulation Study of Aircraft Flight Path Reconstruction. Transport.
2002;
XVII
:
103-107
.
-
SF
Thomsen.
Atopic dermatitis: natural history, diagnosis, and treatment. ISRN allergy 2014.
2014
.
-
MA
Kang,
SY
Choung.
Solanum tuberosum L. cv Hongyoung extract inhibits 2, 4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice. Molecular medicine reports.
2016;
14
:
3093-3103
.
View Article PubMed PMC Google Scholar -
K
Osinka,
K
Dumycz,
B
Kwiek,
W
Feleszko.
Novel Therapeutic Approaches to Atopic Dermatitis. Archivum Immunologiae et Therapiae Experimentalis.
2017
.
-
D
Ezzo.
Treatment and managed care issues of atopic dermatitis. The American journal of managed care.
2017;
23
:
S124-S131
.
PubMed Google Scholar -
WW
Carr.
Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Pediatric Drugs.
2013;
15
:
303-310
.
View Article PubMed PMC Google Scholar -
H
Kang,
CH
Lee,
JR
Kim,
JY
Kwon,
MJ
Son,
JE
Kim,
KW
Lee.
Theobroma cacao extract attenuates the development of Dermatophagoides farinae-induced atopic dermatitis-like symptoms in NC/Nga mice. Food chemistry.
2017;
216
:
19-26
.
View Article PubMed Google Scholar -
V
Karuppagounder,
S
Arumugam,
RA
Thandavarayan,
R
Sreedhar,
VV
Giridharan,
K
Watanabe.
Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug discovery today.
2016;
21
:
632-639
.
View Article PubMed Google Scholar -
EJ
Choi,
ZY
Park,
EK
Kim.
Chemical Composition and Inhibitory Effect of Lentinula edodes Ethanolic Extract on Experimentally Induced Atopic Dermatitis in Vitro and in Vivo. Molecules.
2016;
21
:
993
.
View Article Google Scholar -
Y
Jiang,
W
Ma.
Assessment of Neutrophil-to-Lymphocyte Ratio and Platelet-to- Lymphocyte Ratio in Atopic Dermatitis Patients. Medical science monitor: international medical journal of experimental and clinical research.
2017;
23
:
1340
.
View Article PubMed PMC Google Scholar -
R
Sahoo,
PJS
Jacob,
S
Sahoo.
Biomedical application of green biopolymer guar gum. J Pharm Biomed Sci.
2013;
35
:
1783-87
.
-
T
Shaikh,
SS
Kumar.
Pharmaceutical and pharmacological profile of guar gum an overview. International Journal of Pharmacy and Pharmaceutical Sciences.
2011;
3
:
38-40
.
-
R
Hartemink,
SE
Schoustra,
FM
Rombouts.
Degradation of guar gum by intestinal bacteria. Bioscience and microflora.
1999;
18
:
17-25
.
View Article Google Scholar -
SK
Ghosh,
F
Abdullah,
A
Mukherjee.
Fabrication and fluorescent labeling of guar gum nanoparticles in a surfactant free aqueous environment. Materials Science and Engineering: C.
2015;
46
:
521-529
.
View Article PubMed Google Scholar -
R
Lundberg,
SK
Clausen,
W
Pang,
DS
Nielsen,
K
Möller,
KE
Josefsen,
AK
Hansen.
Gastrointestinal microbiota and local inflammation during oxazolone-induced dermatitis in BALB/cA mice. Comparative medicine.
2012;
62
:
371-380
.
PubMed PMC Google Scholar -
SK
Ghosh,
F
Abdullah,
A
Mukherjee.
Fabrication and fluorescent labeling of guar gum nanoparticles in a surfactant free aqueous environment. Materials Science and Engineering: C.
2015;
46
:
521-529
.
View Article PubMed Google Scholar -
P
Bainbridge.
Wound healing and the role of fibroblasts. Journal of wound care.
2013;
22
.
-
D
Jenerowicz,
M
Czarnecka-Operacz,
W
Silny.
Peripheral blood eosinophilia in atopic dermatitis. ACTA DERMATOVENEROLOGICA ALPINA PANONICA ET ADRIATICA.
2007;
16
:
47
.
-
FT
Liu,
H
Goodarzi,
HY
Chen.
IgE, mast cells, and eosinophils in atopic dermatitis. Clinical reviews in allergy immunology.
2011;
41
:
298-310
.
View Article PubMed Google Scholar -
A
Mócsai.
Diverse novel functions of neutrophils in immunity, inflammation, and beyond. Journal of Experimental Medicine.
2013;
210
:
1283-1299
.
View Article PubMed PMC Google Scholar -
J
Kim,
J
Lee,
S
Shin,
A
Cho,
Y
Heo.
Molecular Mechanism of Atopic Dermatitis Induction Following Sensitization and Challenge with 2, 4-Dinitrochlorobenzene in Mouse Skin Tissue. Toxicological Research.
2018;
34
:
7-12
.
View Article PubMed PMC Google Scholar -
R
Pathak,
SK
Singh,
M
Singh,
A
Henry.
Molecular assessment of genetic diversity in cluster bean (Cyamopsis tetragonoloba) genotypes. Journal of genetics.
2010;
89
:
243-246
.
View Article PubMed Google Scholar
Comments

Downloads
Article Details
Volume & Issue : Vol 5 No 5 (2018)
Page No.: 2305-2325
Published on: 2018-05-22
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 11371 times
- Download PDF downloaded - 1739 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress