Abstract
Introduction: Aging is a physiological process accompanied by cognitive decline, particularly in memory deterioration. D-galactose is a reducing monosaccharide which, if systemically exposed, causes accelerated senescence in several organs and is widely being used as an ideal agent to induce brain aging in animal models. Goat milk is a food of high nutritional value which has been demonstrated to possess strong antioxidant and anti-inflammatory properties. However, thus far, little is known of its possible effects on the brain, especially on memory during aging. The present study examined the efficiency of goat milk supplementation on memory performance in a D-galactose induced aging rat model.
Methods: Fifty-two male Sprague Dawley rats were randomly divided into four groups: 1) control group, 2) goat milk treated group, 3) D-galactose treated group, and 4) goat milk plus D-galactose treated group. D-galactose (120 mg/kg subcutaneously) and/or goat milk (1 g/kg orally) were administered continuously for six weeks, preceded and followed by novel object recognition and T-maze tests.
Results: Prior to goat milk and D-galactose administration, there was no significant difference (p>0.05) in memory performance among all groups. Six weeks of D-galactose administration significantly decreased (p<0.001) short-term, long-term and spatial memory performance. Goat milk supplementation in the D-galactose induced rats managed to protect against memory decline, as exhibited by significantly higher (p<0.0001) short-term, long-term and spatial memory performance of the D-galactose plus goat milk treated group, compared to the D-galactose treated group.
Conclusion: In conclusion, goat milk possesses memoryenhancing effects and, hence. may be useful in protecting against age-related memory deficits.
Introduction
The process of physiological aging is well-known to eventually lead to cognitive decline. With the increase in life expectancy there is an equal rise in the prevalence of cognitive decline and memory loss. The cognitive changes associated with aging predominantly include difficulty in retrieving memories1. Memory alteration is the most common and sought upon cognitive impairment accompanied with aging. Aging itself is considered as the most eminent risk factor for loss in cognitive function amongst older adults2, thereby contributing to poor memory performance.
D‐galactose (D-gal) is a reducing sugar that occurs naturally in the body in small quantities. Excess amounts of D-gal in the body may increase the quantity of reactive oxygen species (ROS) and advanced glycation end products (AGE), which cause considerable brain oxidative damage leading to memory impairment3. An injection of D-gal was first discovered to cause neurological impairments by researchers in China4. Since then, other studies have concluded that D-gal effectively mimics cognitive impairment and characters of the natural brain aging process and, thus, causes significant learning and memory impairment6, 5. It was also discovered that animals treated with D-gal essentially resemble their naturally-aged control counterparts of 16- to 24-months-old7; hence, it is widely considered as an ideal agent for induction of aging in animal models.
Goat milk has high nutritional value and plays a vital role in human nutrition. It is known to contain proteins, vitamins, and fatty acids that possess immense biological merit9, 8. Goat milk possesses higher levels of calcium, potassium and phosphorus- compared to both human and cow milk- and a substantially higher protein concentration compared to human milk11, 10. It is hypoallergic and its small fat globules make it easily digestible11. Thus, due to its composition, it is widely used as a functional food to ameliorate health status as well as reduce the risk of disease development. Moreover, goat milk contains several bioactive peptides with potent antioxidant capacity12. Despite various merits of adding goat milk to diet, there have been very few studies which have explored the role of goat milk in memory and learning, and particularly, during aging.
The aim of this study was to determine memory-enhancing effects of goat milk supplementation in a D-galactose-induced aging rat model. Three types of memory were evaluated, including short-term recognition, long-term recognition, and spatial memory. Specifically, this study aimed to evaluate the effects before and after six weeks of D-gal and/or goat milk treatment on the different types of memory, as well as to compare if there were differential effects across the groups.
Methods
Study design
A randomized controlled trial was conducted with a total of 52 male Sprague-Dawley rats (250-300 grams) approximately 2-months of age. The rats were randomly and equally dividing into four groups as follows (n = 13 per group) : i) control group rats- orally and subcutaneously administered with normal saline, ii) goat milk treated rats- orally administered with 1g/kg goat milk and subcutaneously injected with normal saline, iii) D-gal treated rats- orally administered with normal saline and subcutaneously injected with 120 mg/kg D-gal, and iv) D-gal and goat milk treated rats- orally administered with goat milk and subcutaneously injected with D-gal concomitantly. D-gal (Sigma-Aldrich, USA) and goat milk (Salic Goat, Muazz Marketing, Malaysia) were administered continuously for a period of six weeks, preceded and followed by behavioral tests. The experimental protocol was approved by the Animal Ethics Committee, Universiti Sains Malaysia (USM/IACUC/2017/(109) (879).
Power analysis/ Determination of sample size
In order to determine an adequate sample size, power analysis was conducted in G*Power version 3.1 for a one-way ANOVA with four groups13. Type 1 error probability (α) and the power of the study were set at 0.05 and 80%, respectively. Following the guidelines of Cohen14, an effects size of 0.50 was chosen. The calculated sample size was 12 rats per group. A dropout of 10% was expected, and therefore, the final sample size was 13 rats per group.
Learning and memory tests
Novel object recognition test (NORT)
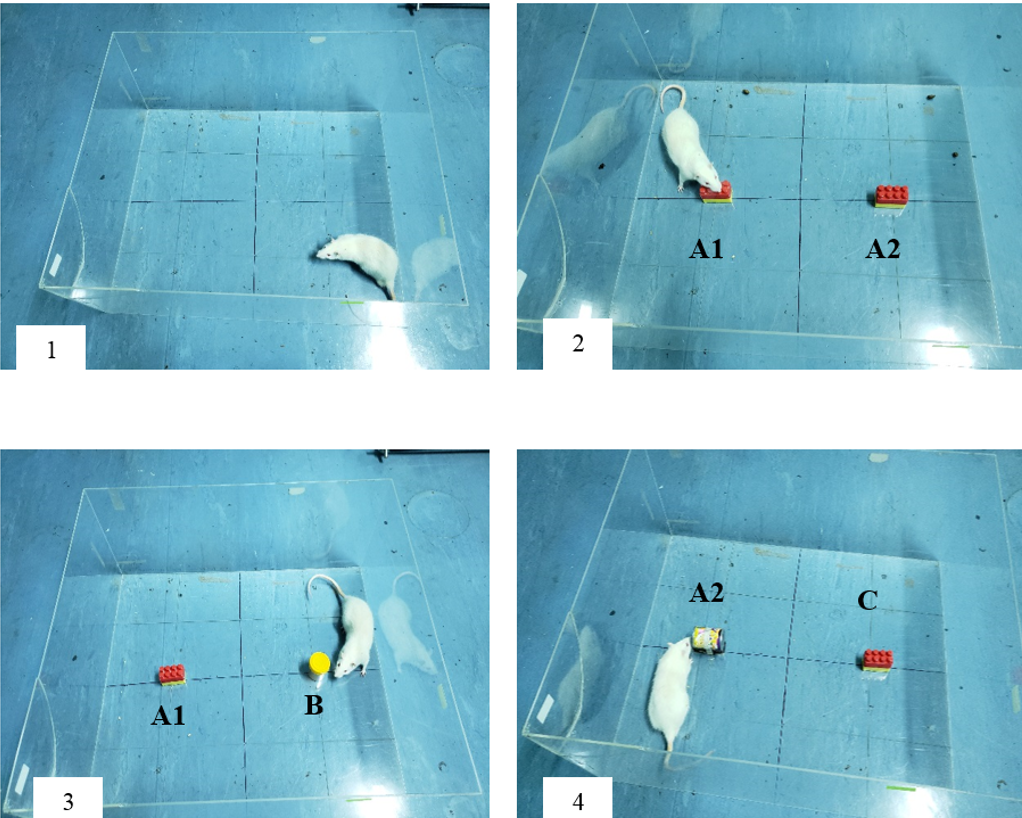
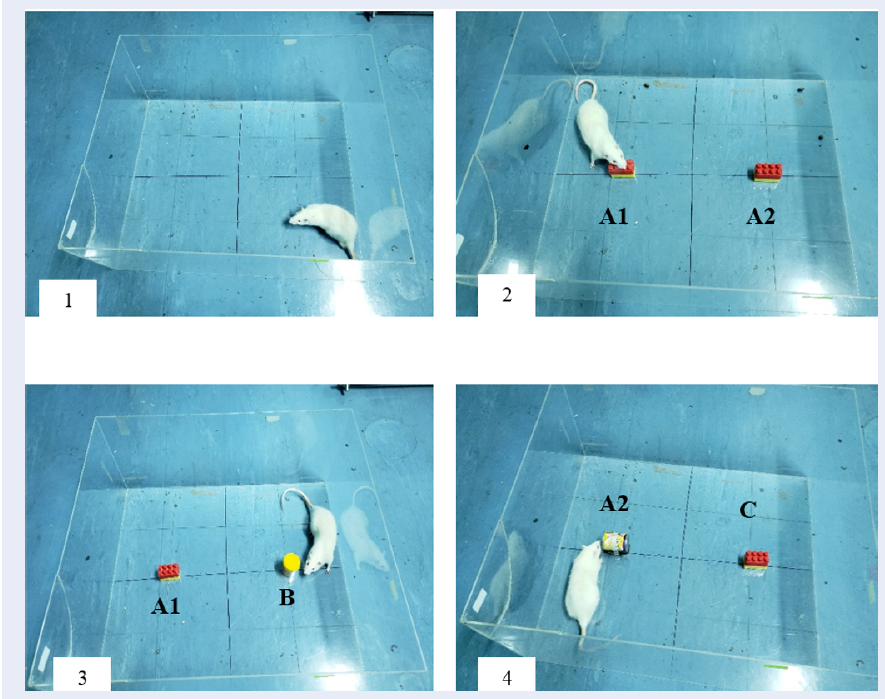
The principle of novel object recognition test is based on the innate preference of rats to explore novel objects as compared to familiar objects15. The protocol used to perform NORT was based on the guidelines provided by previous research studies16. The test was carried out in a transparent open apparatus (as shown in Figure 1). The test began by placing the rat in the empty apparatus for a duration of 10 minutes on the first two days. On the third day, two similar objects (A1 and A2) were placed in the open field at an equal distance from each other and from the walls. The rat was placed inside the open field facing away from the objects for 5 minutes. Then, 2 hours later, one of the familiar objects was replaced with a novel object (B) and the rat was placed back in the arena to explore for 5 minutes as a test for short-term memory (STM). The time spent to explore either objects was recorded carefully. To test for long-term memory (LTM), after 24 hours, the previous novel object (B) was replaced with a new novel object (C) and its place was swapped with the familiar object to eliminate bias. Again, the rat was placed in the open field for 5 minutes and the time to explore both objects was recorded. In order to score for STM and LTM, discrimination index (DI) was calculated by dividing the difference of time used to explore novel and familiar objects by the sum of time spent to explore both novel and familiar objects for each rat. The value for DI ranged from -1 to +1, with -1 representing poor memory and +1 representing excellent memory.
T- maze
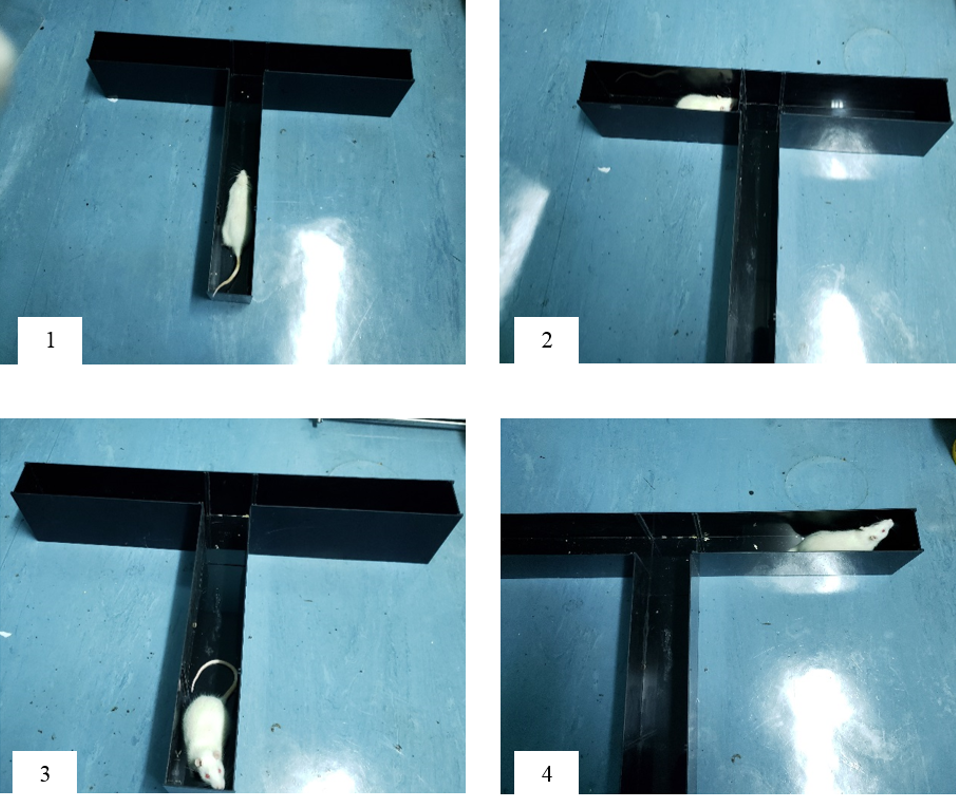
In order to test for spatial memory performance, a spontaneous alteration protocol using a T-maze was followed17. The test began with gently putting the animal in the start arm and allowing it to choose a goal arm (Figure 2). Once the goal arm was chosen, the rat was confined to the chosen arm by quietly sliding the guillotine door down for 30 seconds. After 30 seconds, the animal was gently removed from the maze and the guillotine door was lifted. Then, the animal was placed back in the maze facing away from the goal arms and allowed to choose one of the goal arms. If the animal chose the other arm, as compared to the arm chosen in the first time, the trail was considered successful and a score of 1 was given. If the rat entered the same arm as the one chosen initially, the trial was considered unsuccessful and a score of zero was given. A total of 5 trials was conducted for each rat and the score was given out of 5. The principle of alternation is based on the fact that the animal prefers visiting the less recently visited arm, thus implying that it would need to recall which was the last arm it visited and thus choose the novel arm18.
Statistical analyses
Statistical analysis for all the data was performed using SPSS version 24. Repeated measures ANOVA with pairwise comparison with Bonferroni correction was used to determine any changes in the memory performance pre- and six-weeks post- intervention/treatment within the groups. Before applying the test, assumptions of normality, homogeneity of variances, and compound symmetry were checked and were fulfilled. One-way ANOVA with post-hoc Tukey test was used to analyze any significant changes in memory performance between the groups after six weeks of D-gal and/or goat milk treatment. Before applying the test, the data was tested for normal distribution using the normality test while homogeneity of variance was measured using Levene's test. Results were presented as mean and standard error of mean (S.E.M). Probability values that were less than 5% (p˂0.05) were considered as statistically significant.
| Time | Treatment group | Mean STM | 95% Confidence interval |
|---|---|---|---|
| Pre-treatment | Control | .478 | .360 - .595 |
| Goat milk | .472 | .354 - .589 | |
| D-galactose | .512 | .400 - .625 | |
| D-gal+Goat milk | .521 | .408 - .633 | |
| Post-treatment | Control | .447 | .358 - .535 |
| Goat milk | .625* | .537 - .713 | |
| D-galactose | -.272* | -.357 - -.187 | |
| D-gal+Goat milk | .511$ | .426 - .596 |
Results
Short-term memory performance
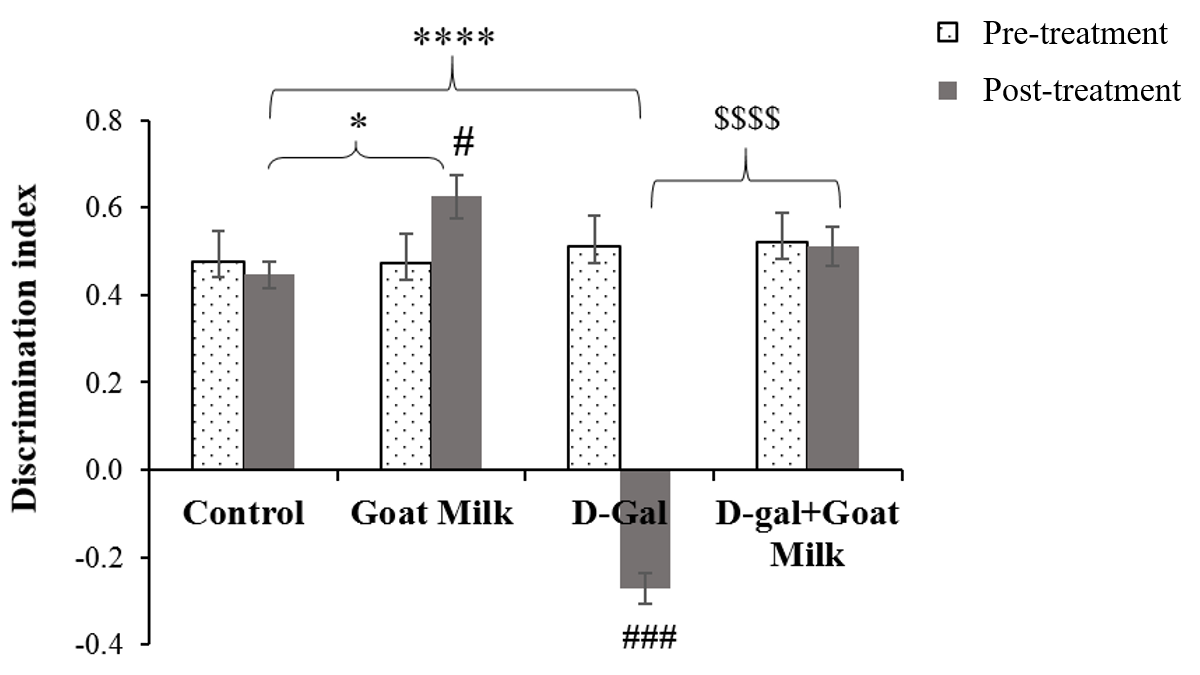
There was a significant difference in mean STM within the treatment groups based on time (F (1, 46) = 7.77, p<0.01). The analyses were followed by pairwise comparison with confidence interval adjustment by Bonferroni correction. The pairwise comparison revealed a significant effect of D-gal on STM pre- and post-treatment (F (1, 12) = 214.405, p ˂0.001). There was a significant improvement in STM among normal rats after six weeks of goat milk administration (F (1, 11) = 5.235, p˂0.05). There was no significant difference in STM between pre- and post-treatment in D-gal rats treated with goat milk, thereby suggesting the protective effect of goat milk against harmful effects of D-gal (F (1,12) = 0.920, p ˃0.05) (Table 1). One-way ANOVA revealed that the mean STM of the goat milk group was significantly higher (p ˂ 0.05) compared to the control group, whereas the mean STM of the D-gal group was significantly lower (p ˂ 0.0001) than that of the control group. The D-gal treated rats supplemented with goat milk exhibited significantly higher mean STM than the D-gal group (p < 0.0001) (Figure 3).
Long-term memory performance
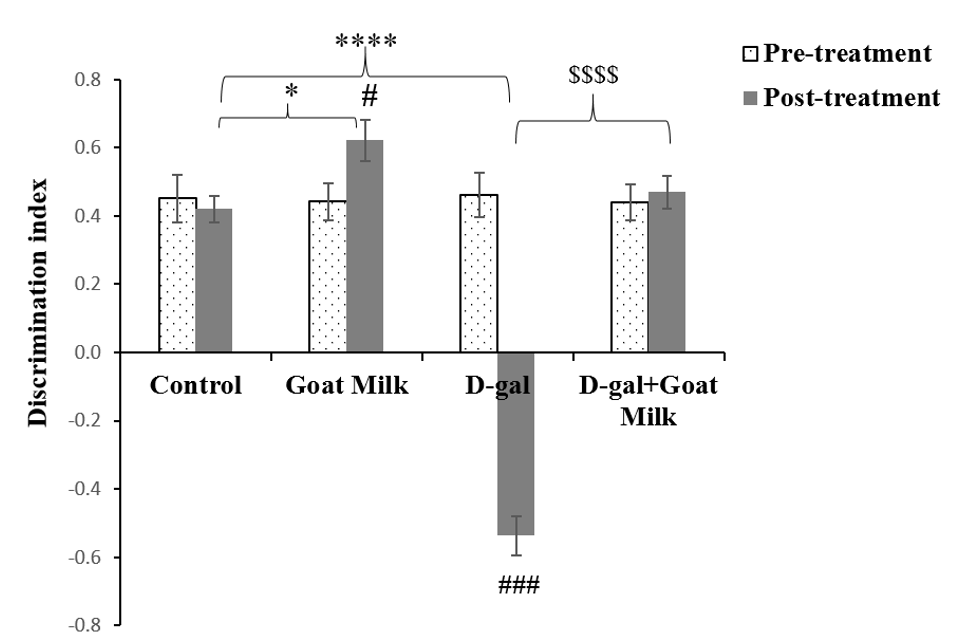
There was a significant difference in mean LTM within the treatment groups based on time (F (1, 46) = 7.53, p<0.01). The analyses were followed by pairwise comparison with confidence interval adjustment by Bonferroni correction. The pairwise comparison revealed a significant effect of D-gal on LTM pre- and post-treatment (F (1, 12) = 109.787, p˂0.001). There was a significant improvement in LTM among normal rats after six weeks of goat milk administration (F (1, 11) = 4.696, p˂0.05). There was no significant difference in LTM between pre- and post-treatment in D-gal rats treated with goat milk, thereby suggesting the protective effect of goat milk against harmful effects of D-gal (F (1,12) = 0.139, p˃0.05) (Table 2). One-way ANOVA demonstrated that the mean LTM of the goat milk group was significantly different (p<0.05) than the control group, whereas the mean LTM of the D-gal group was significantly lower (p<0.0001) than that of the control group. The D-gal treated rats supplemented with goat milk exhibited significantly higher mean LTM than the D-gal group (p<0.0001) (Figure 4).
| Time | Treatment group | Mean LTM | % Confidence interval |
|---|---|---|---|
| Pre-treatment | Control | .451 | .335 - .567 |
| Goat milk | .442 | .326 - .558 | |
| D-galactose | .462 | .351 - .574 | |
| D-gal+Goat milk | .438 | .327 - .550 | |
| Post-treatment | Control | .419 | .313 - .525 |
| Goat milk | .622* | .516 - .728 | |
| D-galactose | -.536* | -.638 - -.434 | |
| D-gal+Goat milk | .470$ | .368 - .572 |
Spatial memory performance
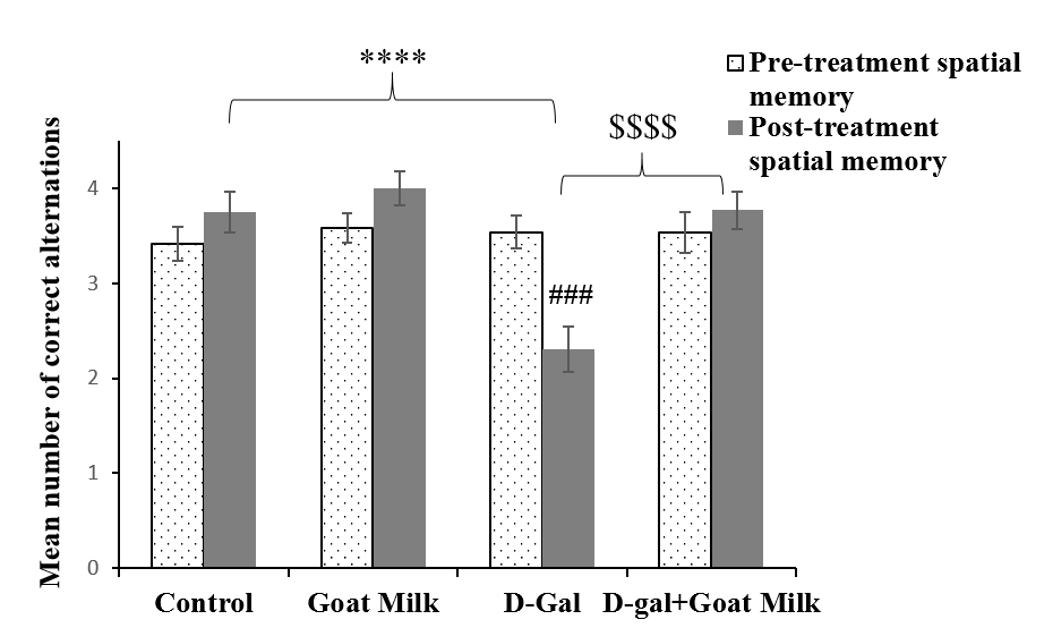
There was no significant difference in mean spatial memory within the treatment groups based on time (F (1, 46) = 0.272, p ˃ 0.05). However, there was a significant difference in time of group interaction (F (3, 46) = 10.53, p < 0.0001). Thus, the analyses were followed by pairwise comparison with confidence interval adjustment by Bonferroni correction. The pairwise comparison revealed a significant effect of D-gal on spatial memory performance of rats pre- and post-treatment (F (1, 12) = 28.444, p˂ 0.001). There was no significant difference between pre- and post-treatment in D-gal rats treated with goat milk (F (1, 12) = 0.806, p˃0.05) (Table 3). One-way ANOVA revealed that the D-gal group exhibited significantly lower mean spatial memory compared to the control group (p<0.0001), whereas the D-gal rats supplemented with goat milk exhibited significantly higher mean spatial memory than the D-gal group (p<0.0001) (Figure 5).
| Time | Treatment group | Mean spatial memory | 95% Confidence interval |
|---|---|---|---|
| Pre-treatment | Control | 3.417 | 3.071-3.763 |
| Goat milk | 3.583 | 3.237-3.929 | |
| D-galactose | 3.538 | 3.206-3.871 | |
| D-gal+Goat milk | 3.538 | 3.206-3.871 | |
| Post-treatment | Control | 3.75 | 3.319-4.181 |
| Goat milk | 4.000 | 3.569-4.431 | |
| D-galactose | 2.308* | 1.893-2.722 | |
| D-gal+Goat milk | 3.769$ | 3.355-4.183 |
Discussion
Aging is an inevitable process which spans various organs of the body of which the brain is the most susceptible to aging. Memory deterioration is the most significant manifestation of brain aging. Decline in memory function results in poor quality of life which adversely affects instrumental activities of daily living (IADLs) and compliance with healthcare19. This renders the older adults vulnerable to development of further diseases and, thus, poor health status. It has been estimated that by the year 2040, almost 81.1 million people will be affected by dementia20. Thus, it is imperative to identify agents or supplements that may help to prevent or delay memory decline.
Goat milk is well-known to possess several biologically useful properties. However, very few research studies have explored its role in the brain, let alone in cognition and memory performance. However, a few studies have been conducted on either goat milk or components richly present in goat milk, as well as their effects on cognition and memory. According to the results of our study, goat milk supplementation successfully improved short- and long-term memory performance of the normal rats. In addition, goat milk was also able to protect against memory decline in the D-gal-treated rats. This was demonstrated by a significant difference in memory performance between the D-gal group and the D-gal group treated with goat milk; the memory performance of the latter group was higher. Interestingly, the memory performance of the D-gal rats treated with goat milk was comparable to that of the normal control rats, suggesting that goat milk has the ability to normalize memory functions.
Xu et al. (2015) reported that goat milk based formula resulted in superior cognitive and spatial ability in weaned Sprague Dawley rats, as compared to control rats and also as compared to rats supplemented with cow milk based formula9. Medeiros et al. (2018) made use of goat whey, which is a by-product of cheese curd production from goat milk, to test memory performance in moderately malnourished male pups; they reported that goat whey was able to enhance memory performance in those pups when compared with the pups in the control group21.
Besides these findings, a few studies have reported the beneficial effects of the individual contents of goat milk on memory and learning. It was reported that the memory-enhancing effects of dried goat whey are attributed to taurine, an amino acid found in goat milk in abundance22. In addition, when added to drinking water, taurine induced recovery of learning and memory in a mouse model of Alzheimer’s disease23.
A few studies have also reported on the beneficial effects, such as on memory performance, of sialic acid (SA), which is abundantly present in goat milk. The SA profile of goat milk closely matches that of human milk24. A positive correlation between SA present in food source and greater cognitive development in animals has also been reported25. Researchers have demonstrated that rat pups performed better at the memory and learning tests when they consumed SA26. Furthermore, piglets that were administered with SA from a food source exhibited improved learning and memory, when compared to controls, as assessed by an 8-arm radial maze25.
Thus, it may be postulated that the memory-enhancing property of goat milk is attributed to SA. In a study by Kanato et al., (2008)27 it was discovered that a homopolymer of SA, known as polysialic acid (polySia), bound directly with a brain-derived neurotrophic factor (BDNF) dimer to form a large complex. This complex was discovered to be responsible for enhancing BDNF expression by binding to the BDNF receptors, TrkB and p75NTR. The complex formation of BDNF with polySia was also accountable for upregulation of growth and survival of neuronal cells. Since BDNF plays an imperative role in memory performance, it may be that by this mechanism, SA improves memory.
Another component abundantly present in goat milk is conjugated linoleic acid (CLA). A research study demonstrated that when CLA was richly present in the maternal diet, it led to enhanced brain development and function in the rat progeny28. This memory-enhancing potential of CLA may be due to its strong antioxidant capacity. Since it is well-known that oxidative stress leads to neuronal loss and memory impairment, and thus to brain aging29, the antioxidant properties of CLA may contribute to its neuroprotective effects.
World-wide, several health and nutritional organizations stress upon the importance of daily consumption of dairy products to attain optimal health; they also strongly recommend including dairy products in one’s diet30. In this study, we demonstrated that goat milk was able to protect against memory impairment caused by D-gal. However, the results of our study only explored the effects of whole goat milk, rather than its individual constituents, on memory performance. The use of whole goat milk renders it ambiguous as to which component of goat milk was responsible for the preservation of memory function. Moreover, the mechanism by which goat milk caused improvement in memory function was also not explored. There are various factors responsible for memory decline, such as oxidative stress, neuronal loss, inflammation, and decrease in brain neurotrophic factors, all of which may be potential mechanisms of action of goat milk. Further studies in these aspects may reveal useful information regarding the role of goat milk in memory preservation.
Conclusions
Considering the results of this study and those from previous reported studies, we conclude that the addition of goat milk to the diet- early on or during old age- may result in protection from memory decline. Thus, goat milk supplementation to diet may eventually lead to better memory performance and, thus, better quality of life.
Abbreviations
D-gal: D-galactose
ROS: Reactive oxygen species
AGE: Advanced glycation end products
NORT: Novel object recognition test
STM: Short-term memory
LTM: Long-term memory
DI: Discrimination index
SEM: Standard error of mean
IADL’s: Instrumental activities of daily living
SA: Sialic acid
CLA: Conjugated linoleic acid
Competing Interests
The authors declare no conflicts of interest.
Authors' Contributions
A.S. carried out the experiments, analysed the data and wrote the paper with input from all authors. K.F.A. designed and supervised the project. R.Z. helped supervise the project. C.B.A.A. partially funded the project. U.R. helped with the experiments.
References
-
Tromp
D.,
Dufour
A.,
Lithfous
S.,
Pebayle
T.,
Després
O.,
Episodic memory in normal aging and Alzheimer disease: insights from imaging and behavioral studies. Ageing Res Rev.
2015;
24
:
232-62
.
PubMed Google Scholar -
Bishop
N.A.,
Lu
T.,
Yankner
B.A.,
Neural mechanisms of ageing and cognitive decline. Nature.
2010;
464
(7288)
:
529-35
.
PubMed Google Scholar -
Fan
S.H.,
Zhang
Z.F.,
Zheng
Y.L.,
Lu
J.,
Wu
D.M.,
Shan
Q.,
Troxerutin protects the mouse kidney from d-galactose-caused injury through anti-inflammation and anti-oxidation. Int Immunopharmacol.
2009;
9
(1)
:
91-6
.
PubMed Google Scholar -
Xu F, editor Sub-acute toxicity of D-galactose. Proceedings of the Second National Conference on Aging Research; 1985..
.
-
Cui
X.,
Zuo
P.,
Zhang
Q.,
Li
X.,
Hu
Y.,
Long
J.,
Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-α-lipoic acid. J Neurosci Res.
2006;
84
(3)
:
647-54
.
PubMed Google Scholar -
Lu
J.,
Zheng
Y.L.,
Luo
L.,
Wu
D.M.,
Sun
D.X.,
Feng
Y.J.,
Quercetin reverses D-galactose induced neurotoxicity in mouse brain. Behav Brain Res.
2006;
171
(2)
:
251-60
.
PubMed Google Scholar -
Song
X.,
Bao
M.,
Li
D.,
Li
Y.M.,
Advanced glycation in D-galactose induced mouse aging model. Mech Ageing Dev.
1999;
108
(3)
:
239-51
.
PubMed Google Scholar -
Soares
J.K.,
de Melo
A.P.,
Medeiros
M.C.,
Queiroga
R.C.,
Bomfim
M.A.,
Santiago
E.C.,
Anxiety behavior is reduced, and physical growth is improved in the progeny of rat dams that consumed lipids from goat milk: an elevated plus maze analysis. Neurosci Lett.
2013;
552
:
25-9
.
PubMed Google Scholar -
Xu
M.,
Wei
L.,
Dai
Z.,
Zhang
Y.,
Li
Y.,
Wang
J.,
Effects of goat milk-based formula on development in weaned rats. Food Nutr Res.
2015;
59
(1)
:
28610
.
PubMed Google Scholar -
Silanikove
N.,
Leitner
G.,
Merin
U.,
Prosser
C.,
Recent advances in exploiting goat's milk: quality, safety and production aspects. Small Rumin Res.
2010;
89
(2-3)
:
110-24
.
-
Park
Y.W.,
Goat milk–chemistry and nutrition. Handbook of milk of non‐bovine mammals 2017.
Google Scholar -
De Gobba
C.,
Espejo-Carpio
F.J.,
Skibsted
L.H.,
Otte
J.,
Antioxidant peptides from goat milk protein fractions hydrolysed by two commercial proteases. Int Dairy J.
2014;
39
(1)
:
28-40
.
-
Faul
F.,
Erdfelder
E.,
Lang
A.G.,
Buchner
A.,
GPower 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods.
2007;
39
(2)
:
175-91
.
PubMed Google Scholar -
Cohen
J.,
Statistical power analysis for the behaviors scienceLaurence Erlbaum Associates Publishers: New Jersey; 1988.
Google Scholar -
Antunes
M.,
Biala
G.,
The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process.
2012;
13
(2)
:
93-110
.
PubMed Google Scholar -
Ennaceur
A.,
Delacour
J.,
A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res.
1988;
31
(1)
:
47-59
.
PubMed Google Scholar -
Deacon
R.M.,
Rawlins
J.N.,
T-maze alternation in the rodent. Nat Protoc.
2006;
1
(1)
:
7-12
.
PubMed Google Scholar -
Hughes
R.N.,
The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev.
2004;
28
(5)
:
497-505
.
PubMed Google Scholar -
Woods
S.P.,
Weinborn
M.,
Li
Y.R.,
Hodgson
E.,
Ng
A.R.,
Bucks
R.S.,
Does prospective memory influence quality of life in community-dwelling older adults?. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn.
2015;
22
(6)
:
679-92
.
PubMed Google Scholar -
Ferri
C.P.,
Prince
M.,
Brayne
C.,
Brodaty
H.,
Fratiglioni
L.,
Ganguli
M.,
Alzheimer's Disease International
Global prevalence of dementia: a Delphi consensus study. Lancet.
2005;
366
(9503)
:
2112-7
.
PubMed Google Scholar -
Medeiros
L.B.,
Vitor-de-Lima
S.M.,
Lira Benevides
R.D.,
do Egypto Queiroga
R.C.,
Araújo Guedes
R.C.,
Neonatal administration of goat whey modulates memory and cortical spreading depression in rats previously suckled under different litter sizes: possible role of sialic acid. Nutr Neurosci.
2018;
21
(2)
:
108-15
.
PubMed Google Scholar -
Wenting
L.,
Ping
L.,
Haitao
J.,
Meng
Q.,
Xiaofei
R.,
Therapeutic effect of taurine against aluminum-induced impairment on learning, memory and brain neurotransmitters in rats. Neurol Sci.
2014;
35
(10)
:
1579-84
.
PubMed Google Scholar -
Kim
H.Y.,
Kim
H.V.,
Yoon
J.H.,
Kang
B.R.,
Cho
S.M.,
Lee
S.,
Taurine in drinking water recovers learning and memory in the adult APP/PS1 mouse model of Alzheimer's disease. Sci Rep.
2014;
4
(1)
:
7467
.
PubMed Google Scholar -
Kiskini
A.,
Difilippo
E.,
Oligosaccharides in goat milk: structure, health effects and isolation. Cellular and molecular biology (Noisy-le-Grand, France).
2013;
59
(1)
:
25-30
.
-
Wang
B.,
Yu
B.,
Karim
M.,
Hu
H.,
Sun
Y.,
McGreevy
P.,
Dietary sialic acid supplementation improves learning and memory in piglets. Am J Clin Nutr.
2007;
85
(2)
:
561-9
.
PubMed Google Scholar -
Wang
B.,
Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv Nutr.
2012;
3
(3)
:
465-72
.
PubMed Google Scholar -
Kanato
Y.,
Kitajima
K.,
Sato
C.,
Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology.
2008;
18
(12)
:
1044-53
.
PubMed Google Scholar -
Soares
J.K.,
Rocha-de-Melo
A.P.,
Medeiros
M.C.,
Queiroga
R.C.,
Bomfim
M.A.,
de Souza
A.F.,
Conjugated linoleic acid in the maternal diet differentially enhances growth and cortical spreading depression in the rat progeny. Biochim Biophys Acta.
2012;
1820
(10)
:
1490-5
.
PubMed Google Scholar -
Floyd
R.A.,
Hensley
K.,
Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging.
2002;
23
(5)
:
795-807
.
PubMed Google Scholar -
Bishop-MacDonald
H.,
Dairy food consumption and health: State of the science on current topics. J. Am. College Nutr.
2005;
24
(6)
:
525-535
.
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 1 (2020)
Page No.: 3563-3571
Published on: 2020-01-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6820 times
- Download PDF downloaded - 1929 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress