Abstract
Aim: To evaluate pain relief, anti-inflammatory and hypouricemic effects of GT1 tablets on experimental animals.
Method: GT1 at the doses of 22.32 g/kg/day and 66.96 g/kg/day were evaluated for its analgesic effect in three models (hot plate, pain threshold, and acetic acid-induced writhing), its chronic anti-inflammatory effect in the granulomatous reaction model, and its hypouricemic effect in potassium oxonate-induced hyperuricemic mice. Acute anti-inflammatory effects of GT1 at the doses of 11.16 g/kg/day and 33.48 g/kg/day were evaluated in rats with two models: carrageenin-induced paw edema and peritonitis.
Results: GT1 prolonged the temperature reaction time on the hot plate (22.73 s and 20.37 s at both doses of 22.32 g/kg and 66.96 g/kg, respectively, compared to 16.96 s in control group), reduced the number of acid acetic-induced writhing effects, decreased the weight of granulomas, and decreased the level of acid uric in blood and urine (p < 0.05). GT1 caused a significant reduction in paw edema after subplantar injection of carrageenan in rats (p < 0.05). Moreover, there was a substantial decline of GT1 at the dose of 11.16 g/kg/day in terms of the volume and the quantity of protein in the inflammation fluid of the peritonitis model (p < 0.05).
Conclusion: GT1 at both doses of 11.16 g/kg/day and 33.48 g/kg/day posed acute anti-inflammatory effects on rats. GT1 at both doses of 22.32 g/kg/day and 66.96 g/kg/day exerted analgesic, chronic anti-inflammatory and hypouricemic effects on mice.
INTRODUCTION
Gout, a consequence of precipitation of monosodium urate crystals in a joint space, is a common chronic condition that mainly causes painful joint inflammation in the first metatarsophalangeal joint1, 2. Gout is increasingly common given the popularity of poor dietary habit and unhealthy lifestyles, as well as the increase in prevalence of obesity and metabolic syndromes3. According to European and American regional statistics, the prevalence of gout was 3-6% among male populations and 1-2% among females4.
Treatments for gout have been well-investigated, and include non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids. However, these medications are related to adverse effects5. The development of novel therapeutic natural agents is, therefore, important to improve the disease condition6, 7. In a previous review, traditional herbal medicine was found to have similar efficacy in gout treatment compared to Western medications, with respect to serum uric acid, C reactive protein, erythrocyte sedimentation rate and overall clinical response8. Moreover, traditional medicine was more advantageous, compared to Western medicine, regarding adverse drug reaction control8. Therefore, developing traditional herbal medicine regimes for the treatment of gout- along with traditional Western medicine- has great potential and should be considered.
The prevalence of gout in Vietnam is about 0.14% of the general population and is projected to increase significantly due to the rapid urbanization and lifestyle changes4, 9. In order to combine modern medicine with traditional medicine, GT1 remedy was developed for gout treatment. GT1 film-coated tablets are prepared from natural materials, including Atractylodes lancea (Thunb.) DC, Phellodendron chinense (Schneid), Achyranthes bidentata (Blume), Lonicera japonica (Thunb.), Anemarrhena asphodeloides (Bunge), Talcum, Milletia reticulata (Benth), Paeonia lactiflora (Pall), Plantago asiatica L, Coix lachryma-jobi L., Dioscorea tokoro (Mahino), and Clematis simensis (Osheck). In the present study, we aimed to validate the anti-inflammatory, antinociceptive activities and hypouricemic effects of GT1 on animal models.
METHODS
The preparation of GT1 film-coated tablets
GT1 with standard basis was provided by Traditional Medicine Hospital, Ministry of Public Security, Vietnam. The standard for tablet manufacturing process complied with the current standard basis of Traditional Medicine Hospital, Ministry of Public Security, Vietnam. GT1 was formulated in the form of film-coated tablets, and each tablet had 0.5 mg which was achieved from 0.75 g Atractylodes lancea (Thunb.) DC, 0.75 g Phellodendron chinense (Schneid), 0.75 g Achyranthes bidentata (Blume), 1.0 g Lonicera japonica (Thunb.), 0.75 g Anemarrhena asphodeloides (Bunge), 0.625 g Talcum, 1.875 g Milletia reticulata (Benth), 1.0 g Paeonia lactiflora (Pall), 0.625 g Plantago asiatica L, 1.875 g Coix lachryma-jobi L., 1.0 g Dioscorea tokoro (Makino), and 0.625 g Clematis simensis (Osheck). atients were given orally 4 tablets per day for twice-daily dosing.
Drugs, chemicals and laboratory equipment
Aspirin was collected from Aspirin tablet with a brand-name 100-mg intestinal-soluble tablets (Traphaco Joint Stock Company, Vietnam). Codeine phosphate was supplied by the Central Institute of Drug Quality Control. Methyl prednisolon (Medrol) 4-mg tablet was from Pfizer, Inc (New York, NY). Potassium oxonate, 1% acetic acid solution, 1% carrageenan solution, and formaldehyde solution met the laboratory standards and were provided by Hanoi Medical University, Vietnam.
Equipment included: hot plate (model DS37 from Ugo-Basile, 21036 Gemonio VA, Italy), Dynamic Plantar Aesthesiometer 37450 pain response machine (Italy), Ueth-Basile Plethysmometer No7250 (from Ugo-Basile, 21036 Gemonio VA, Italy), biochemical testing machine/ semi-automatic XC — 55 chemistry analyzer (China), and Vet abcTM Animal Blood Counter (France). Kits for protein measurement were obtained from Hospitex Diagnostics (Italy), and ABX Minidil LMG blood test solution was from ABX – Diagnostics (78754 Austin, TX).
Experimental animals
In this study, we used Wistar rats (150-200 g in weight) and Swiss mice (20-22 g in weight), regardless of sex. Ten rats/mice were housed in cages at the Department of Pharmacology, Hanoi Medical University, Vietnam for at least 1 week prior to investigation. The cages had standard access to rodent diet and water ad libitum. The study protocol was approved by the Institutional Review Board of the Military Institute of Traditional Medicine, Hanoi, Vietnam (Code 05/QD-VYHCTQD, date 04/01/2017).
Methods
Anti-inflammatory activity
Carrageenan-induced rat paw edema
The animals were divided into four groups of ten rats per cage: Group 1 (Control group) received distilled water at 0.2 mL/10 g; Group 2 (Positive group) received aspirin at a dose of 200 mg/kg; Group 3 received GT1 at a dose of 11.16 g/kg; and Group 4 received GT1 at a dose of 33.48 g/kg.
All the treatments were given continuously for five days. One hour after the first dose, 0.05 mL of 1% carrageenan solution in normal saline was injected into the treated paw. Carrageenan-induced swelling and contralateral feet (injected with 0.9% saline) were measured before carrageenan injection (V0) and at 2 (V2), 4 (V4), 6 (V6) and 24 hours (V24) after the injection. Pedal edema level was evaluated by measuring the left hind paw volume using Plethysmography No 7250 water (Ugo Basile, Italy). Paw thickness was measured using a digital vernier caliper.
Carrageenan-induced peritonitis
Four groups of ten rats were given the same pre-treatments as in the above model. Rats received intraperitoneal injection of 0.05 g carrageenan and formaldehyde (1.5 mL) mixed in 100 mL physiological saline in order to induce inflammation in the peritoneal cavity. All the treatments were given 5 days before the carrageenin and formaldehyde injection. At 24 hours after the injection, the peritoneal fluid was withdrawn and the volume of the fluid, total number of leukocytes, and amount of protein in the peritoneal fluid were counted.
Granuloma method
The animals were divided into 4 groups of ten mice per cage: Group 1 (Control group) received distilled water at 0.2 mL/10 g; Group 2 (Positive group) received methylprednisolone at a dose of 10 mg/kg; Group 3 received GT1 at a dose of 22.32 g/kg; and Group 4 received GT1 at a dose of 66.96 g/kg.
Asbestos fiber weighing 6 mg was sterilized for 1 hour at 120°C and embedded in carrageenan (1%). We aseptically made a subcutaneous tunnel using blunted forceps in the shaved nape skin region of each mice. Each carrageenan-embedded asbestos fiber was then implanted bilaterally in the subcutaneous tunnel. After that, rats were given the treatment with water, drug, or GT1 within 10 days continuously. On the 11th day, the mice were sacrificed. From that point, the pellets encompassed by granuloma tissue were carefully excised and removed from extraneous tissue. The damp weight of the asbestos fiber was taken instantly after expulsion; after that, it was dried at 56°C for 18 h and the net dry weight was evaluated.
Analgesic activity
Hot plate test
The animals were divided into 4 groups of ten mice per cage: Group 1 (Control group) received distilled water at 0.2 mL/10 g; Group 2 (Positive group) received codeine phosphate at a dose of 20 mg/kg; Group 3 received GT1 at a dose of 22.32 g/kg; and Group 4 received GT1 at a dose of 66.96 g/kg.
The hot plate was maintained at 560C. The mice of each arm were put in the container (on the hot plate) to watch their reaction to electrical heat-induced pain. At baseline, we measured the normal reaction of the animals to normal temperature before administration of GT1, water or codeine phosphate. At 30 minutes after taking the intervention, we recorded the response time (in seconds) when the rats/mice licked their fore and/or rear paws, as well as jumped. Animals showing a response time more than 30s or less than 8s were disposed of.
Mechanical sensitivity
Four groups of ten mice each were given same pre-treatments as those in the hot plate model. Paw withdrawal latency in reaction to mechanical stimulation was evaluated with an automated testing gadget, comprising of a steel rod that was pushed against the plantar surface of the paw, with expanding drive until the paw was pulled back (Dynamic Plantar Aesthesiometer 37450, Ugo Basile, Italy). Before administration and 1 hour after the final administration, the paw withdrawal latency was assessed, and the drive required to evoke a paw withdrawal reflex was recorded and measured in grams.
Acetic acid-induced writhing test
Four groups of ten mice were given the same pre-treatments as those in the hot plate model; however, the reference analgesic drug was aspirin (150 mg/kg). On the 5th day, at 1 hour after administration of water, reference drug or GT1, abdominal constriction was induced in mice by intraperitoneal injection of 0.2 mL acetic acid (1%). The number of abdominal constrictions was cumulatively counted over a period of 5 minutes within 30 minutes.
Hypouricemic activity
Animals were divided into five groups (n=10): Group 1 (Control) received distilled water (0.2 mL/10 g) and was injected with CMC-Na (0.5%); Group 2 (Model) received distilled water (0.2 mL/10 g) and was injected intraperitoneally (i.p.) with potassium oxonate; Group 3 was treated with allopurinol (20 mg/kg) and received i.p. injection of potassium oxonate; and Groups 4 and 5 were treated with GT1 at dose of 22.32 g/kg and GT1 66.96 g/kg, respectively, and received i.p. injection of potassium oxonate. Once a day for a period of 7 days, water and drug were given by oral gavage. On the 7th day of the experiment, one hour after treatment, mice were injected with CMC-Na (0.5%) and potassium oxonate (500 mg/kg). The concentrations of acid uric and creatinine in blood and urine were determined.
Data analysis
Data were shown as mean and standard deviation. Data were analyzed using Microsoft Excel software. The levels of significance between the experimental groups and the control groups were made using Student's t-test; a p-value less than 0.05 was considered as significantly different.
RESULTS
Anti-inflammatory activity
Carrageenan-induced rat paw edema
Table 1 showed that GT1 at the dose of 11.16 g/kg caused a significant reduction in paw edema at six hours after the injection of carrageenan (14.84% reduction compared to Group 1) and 24 h after the injection of carrageenan (9.87% reduction compared to Group 1). GT1 at the dose of 33.48 g/kg significantly decreased the degree of pedal edema at 6 h after the injection of carrageenan (15.61% reduction compared to Group 1).
Carrageenan-induced peritonitis
Table 2 illustrates that rats treated with GT1 at the dose of 11.46 g/kg showed significant reductions in the volume of inflammatory fluid, the total number of leukocytes, and the amount of protein in the peritoneal fluids compared with the control group. However, no significant difference was observed between the GT1 33.48 g/kg dose group and the control group (p > 0.05).
Granuloma method
Table 3 shows that GT1 at the dose of 66.96 g/kg reduced the wet and dry weight of granuloma as compared with the control group (p < 0.05). There was no considerable change in the weight of the granuloma in the group treated with GT1 (22.32 g/kg dose), when compared with the control group (p > 0.05).
| Time (h) | % paw volume | |||
| Group 1 (Control) | Group 2 (Aspirin 200 mg/kg) | Group 3 (GT111.16g/kg) | Group 4 (GT133.48g/kg) | |
| After 2h | 20.57 ± 6.09 | 12.37 ± 2.80*** | 24.20 ± 7.83+++ | 32.62 ± 8.67**+++ |
| After 4h | 54.53 ± 16.58 | 30.73 ± 9.18*** | 43.74 ± 11.97+ | 52.11 ± 16.39++ |
| After 6h | 52.65 ± 1214 | 45.13 ± 11.75 | 37.81 ± 9.20** | 37.04 ± 10.79** |
| After 24h | 26.36 ± 5.67 | 23.55 ± 7.95 | 16.49 ± 3.59***+ | 21.66 ± 5.05 |
| Group | Group 1 (Control) | Group 2 (Aspirin 200 mg/kg) | Group 3 (GT1 11.16 g/kg) | Group 4 (GT133.48 g/kg) |
| The volume of fluid (mL/100 g) | 1.34 ± 0.38 | 0.83 ± 0.24** | 0.88 ± 0.27** | 1.94 ± 0,60 |
| Total number of leukocytes (G/L) | 32.48 ± 7.13 | 19.21 ± 6.29** | 18.83 ± 6.98** | 41.62 ± 12.73 |
| The amount of protein (mg/dL) | 14.49 ± 3.06 | 8.83 ± 2.83*** | 8.29 ± 2.70*** | 22.19 ± 6.36** |
| Group | The wet weight ofgranuloma (mg) | The dryweight of granuloma (mg) |
| Group 1: Control | 90.76 ± 18.73 | 18.53 ± 6.23 |
| Group 2: Methylprednisolon 10 mg/kg | 59.23 ± 18.33*** | 12.78 ± 4.08* |
| Group 3: GT1 22.32 g/kg/day | 81.50 ± 22.48+ | 19.40 ± 6.28++ |
| Group 4: GT1 66.96 g/kg/day | 72.70 ± 19.58* | 14.30 ± 3.11* |
| Group | Reaction time (± SD) (s) | |
| Before | After | |
| Group 1: Control | 17.21 ± 4.78 | 16.96 ± 3.46 |
| Group 2: Methylprednisolon 10 mg/kg | 18.87 ± 2.72 | 21.59 ± 4.43* |
| Group 3: GT1 22.32 g/kg/day | 18.86 ± 3.63 | 22.73 ± 3.81** |
| Group 4: GT1 66.96 g/kg/day | 16.70 ± 2.75 | 20.37 ± 3.43* |
Analgesic activity
Hot plate test
Table 4 shows that the treatment with GT1 at both doses of 22.32 g/kg and 66.96 g/kg induced a significant increase in the reaction time on the hot plate, as compared with the control group (22.73 s and 20.37 s, respectively, compared to 16.96 s for control).
Mechanical sensitivity
Table 5 demonstrates that after administration of GT1 for 5 days, GT1 at both doses of 22.32 g/kg and 66.96 g/kg tended to increase the pressure and reaction time as compared with the control group and before the administration. However, the differences were not significant (p > 0.05).
| Group | Pressure (g) | Reaction time (s) | ||
| Before | After | Before | After | |
| Group 1: Control | 9.20 ± 1.52 | 10.29 ± 1.42 | 5.31 ± 0.92 | 5.97 ± 0.87 |
| Group 2: Methylprednisolon 10mg/kg | 9.47 ± 1.70 | 11.81 ± 1.72* | 5.48 ± 1.03 | 6.90 ± 1.08* |
| Group 3: GT1 22.32 g/kg/day | 9.93 ± 2.52 | 10.56 ± 3.30 | 5.75 ± 1.52 | 6.17 ± 1.96 |
| Group 4: GT1 66.96 g/kg/day | 10.24 ± 0.98 | 10.77 ± 2.90 | 5.94 ± 0.60 | 6.25 ± 1.74 |
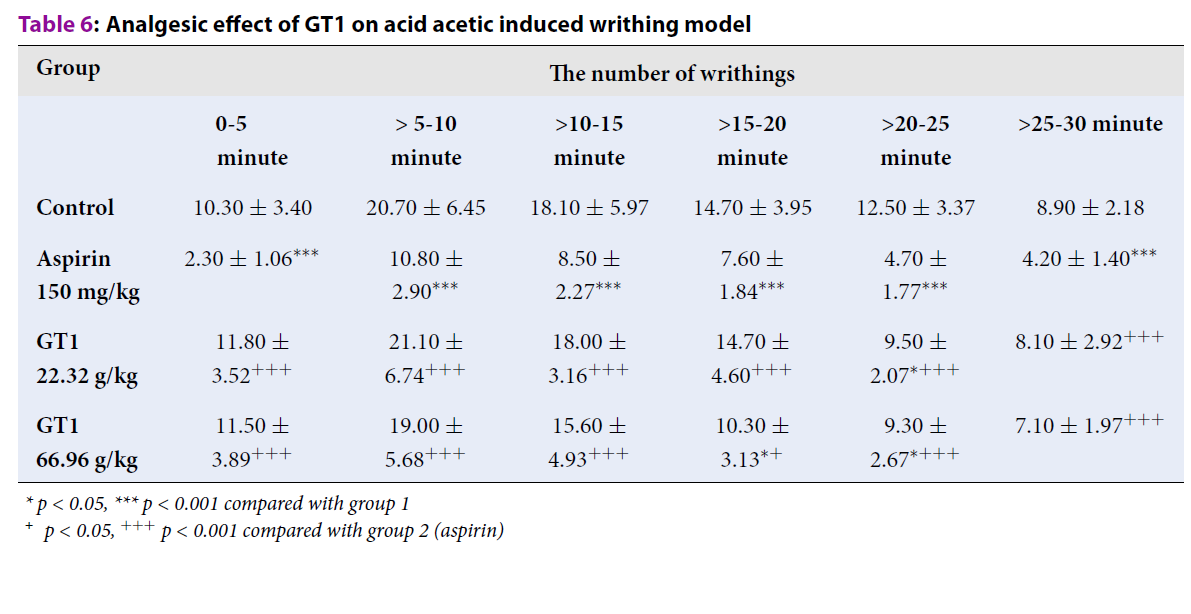
Acid acetic-induced writhing
Table 6 indicates that there was a considerable reduction in the number of writhings in the group treated with GT1 at the dose of 22.32 g/kg at the period of >20-25 minutes, when compared with the control group (p < 0.05). GT1 at the dose of 66.96 g/kg significantly decreased the number of writhings at the periods of >15-20 minutes and >20-25 minutes, as compared with the control group (p < 0.05).
| Group | The number of writhings | |||||
| 0-5 minute | > 5-10 minute | >10-15 minute | >15-20 minute | >20-25 minute | >25-30 minute | |
| Control | 10.30 ± 3.40 | 20.70 ± 6.45 | 18.10 ± 5.97 | 14.70 ± 3.95 | 12.50 ± 3.37 | 8.90 ± 2.18 |
| Aspirin 150 mg/kg | 2.30 ± 1.06*** | 10.80 ± 2.90*** | 8.50 ± 2.27*** | 7.60 ± 1.84*** | 4.70 ± 1.77*** | 4.20 ± 1.40*** |
| GT1 22.32 g/kg | 11.80 ± 3.52+++ | 21.10 ± 6.74+++ | 18.00 ± 3.16+++ | 14.70 ± 4.60+++ | 9.50 ± 2.07*+++ | 8.10 ± 2.92+++ |
| GT1 66.96 g/kg | 11.50 ± 3.89+++ | 19.00 ± 5.68+++ | 15.60 ± 4.93+++ | 10.30 ± 3.13*+ | 9.30 ± 2.67*+++ | 7.10 ± 1.97+++ |
Hypouricemic activity
Table 7 and Table 8 show that GT1 at both doses of 22.32 g/kg and 66.96 g/kg decreased the concentration of acid uric in blood and urine, as compared with the control group. GT1 at both doses of 22.32 g/kg and 66.96 g/kg, however, did not significantly change the concentration of creatinine in blood and urine, when compared with the control group (p > 0.05).
| Group | The concentration of serum acid uric (μmol/mL) | The concentration of urine acid uric (μmol/mL) |
| Group 1 (control) | 16.00 ± 4.71 | 18.70 ± 5.91 |
| Group 2 (model) | 21.70 ± 5.42* | 28.00 ± 8.45* |
| Group 3 (allopurinol 20 mg/kg) | 14.20 ± 3.97ΔΔ | 19.30 ± 6.02Δ |
| Group 4 (GT1 22.32 g/kg) | 16.20 ± 3.97Δ | 14.50 ± 2.59ΔΔΔ |
| Group 5 (GT1 66.96 g/kg) | 17.80 ± 2.15Δ | 17.80 ± 5.75ΔΔ |
| Group | Concentration of creatinine in blood (mg/dL) | Concentration of creatinine in urine (mg/dL) |
| Group 1 (control) | 0.48 ± 0.12 | 5.03 ± 1.25 |
| Group 2 (model) | 0.48 ± 0.15 | 5.28 ± 1.00 |
| Group 3 (allopurinol 20 mg/kg) | 0.48 ± 0.12 | 5.99 ± 1.17 |
| Group 4 (GT1 22.32 g/kg) | 0.53 ± 0.14 | 4.99 ± 0.75 |
| Group 5 (GT1 66.96 g/kg) | 0.55 ± 0.17 | 5.38 ± 0.81 |
DISCUSSION
Acute inflammation has typical symptoms such as pain, swelling, heat or redness. Therefore, measuring swelling degree is a good indicator for experimentally-induced acute inflammation. In our study, oral administration of GT1 was capable of decreasing paw volume of rats, volume of peritoneal fluid, total number of leukocytes, and amount of protein, as well as inhibiting granuloma formation, when compared with the control group. Therefore, GT1 induces a positive effect in animal models of acute and chronic inflammation.
Pain is known to be often associated with inflammation10. In this study, the antinociceptive effect of GT1 was evaluated using in vivo models including the hot plate model, mechanical sensitivity test, and acid acetic-induced nociception test. GT1 at both doses of 22.32 g/kg and 66.96 g/kg significantly increased the reaction time on the hot-plate, tended to increase the pressure and reaction time on the dynamic plantar aesthesiometer, and reduced the number of writhings as compared with the control group. These results indicate that GT1 expresses central and peripheral analgesic activities.
Hyperuricemia could be a major hazardous factor for gout and chronic nephritis in the clinical setting11. In this study, GT1 at both doses of 22.32 g/kg and 66.96 g/kg decreased the concentration of acid uric in blood and urine, when compared with the control group. GT1 at both doses did not significantly change the concentration of creatinine in blood and urine, as compared with the control group. These results indicated that GT1 potentiated the analgesic, anti-inflammatory and hypouricemic effects. This was due to the effect of the main components in GT1. According to the research of Ryu (2010), SKLJI, an extract from Lonicera japonica, is a viable candidate for new anti-inflammatory and analgesic phytomedicine12. WIN-34B, an extract from Lonicera japonica and Anemarrhena asphodeloides (Bunge), exhibited anti-inflammatory and analgesic activities in vitro and in vivo13, 14. He DY and Dai SM (2011) reported on the anti-inflammatory and analgesic activities of Paeonia lactiflora (Pall) in vivo and in vitro15. According to Yang Q (2019), polysaccharides purified from Lonicera japonica expressed anti-hyperuricemic and anti-gout effects through significantly reducing serum uric acid and suppressing xanthine oxidase activity16. Results from the study of Yang Fei (2016) showed that Dioscorea tokoro (Makino) at a dose of 880 mg/kg decreased levels of serum uric acid, increased levels of urine uric acid, and inhibited xanthine oxidase activity in both serum and liver11.
CONCLUSIONS
GT1 at the doses of 11.16 g/kg/day and 33.48 g/kg/day mediated an acute anti-inflammatory effect on rats. GT1 at both doses of 22.32 g/kg/day and 66.96 g/kg/day exerted analgesic, chronic anti-inflammatory and hypouricemic effects on mice.
Abbreviations
NSAIDs: Non-steroidal anti-inflammatory drugs
Acknowledgments
We would acknowledge the support from staff of Traditional Medicine Hospital, Ministry of Public Security, Hanoi, Vietnam.
Author’s contributions
Conceptualization: Pham Ba Tuyen, Truong Thi Huyen. Formal analysis: Pham Ba Tuyen, Truong Thi Huyen, Pham Xuan Phong. Investigation: Truong Thi Huyen, Pham Xuan Phong, Dinh Thi Thu Hang. Methodology: Pham Ba Tuyen, Truong Thi Huyen, Pham Xuan Phong, Nguyen Trong Thong, Dinh Thi Thu Hang, Pham Thi Van Anh. Project administration: Pham Xuan Phong, Nguyen Trong Thong. Writing-original draft: Pham Ba Tuyen, Truong Thi Huyen, Pham Xuan Phong, Nguyen Trong Thong, Dinh Thi Thu Hang, Pham Thi Van Anh. Writing. Review & editing: Pham Ba Tuyen, Truong Thi Huyen, Pham Xuan Phong, Nguyen Trong Thong, Dinh Thi Thu Hang, Pham Thi Van Anh. All authors gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study received funding from the Traditional Medicine Hospital, Ministry of Public Security.
Availability of data and materials
Data and materials used and/or analysed during the current study are available from the corresponding author on reasionable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Hainer
B.L.,
Matheson
E.,
Wilkes
R.T.,
Diagnosis, treatment, and prevention of gout. American family physician.
2014;
90
(12)
:
831-836
.
-
Goodman
L.S.,
Goodman and Gilman's the pharmacological basis of therapeutics. McGraw-Hill New York.
1996
.
-
Ragab
G.,
Elshahaly
M.,
Bardin
T.,
Gout: An old disease in new perspective - A review. Journal of advanced research.
2017;
8
(5)
:
495-511
.
View Article PubMed Google Scholar -
Kuo
C.F.,
Grainge
M.J.,
Zhang
W.,
Doherty
M.,
Global epidemiology of gout: prevalence, incidence and risk factors. Nature reviews Rheumatology.
2015;
11
(11)
:
649-662
.
View Article PubMed Google Scholar -
Soskind
R.,
Abazia
D.T.,
Bridgeman
M.B.,
Updates on the treatment of gout, including a review of updated treatment guidelines and use of small molecule therapies for difficult-to-treat gout and gout flares. Expert opinion on pharmacotherapy.
2017;
18
(11)
:
1115-1125
.
View Article PubMed Google Scholar -
Kabir
I.,
Imtiyaz
A.,
A review on in vivo and in vitro experimental models to investigate the anti-inflammatory activity of herbal extracts. Asian Journal of Pharmaceutical and Clinical Research.
2018;
11
:
29
.
View Article Google Scholar -
Khanna
D.,
Fitzgerald
J.D.,
Khanna
P.P.,
Bae
S.,
Singh
M.K.,
Neogi
T.,
American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken).
2012;
64
(10)
:
1431-1446
.
View Article PubMed Google Scholar -
Zhou
L.,
Liu
L.,
Liu
X.,
Chen
P.,
Liu
L.,
Zhang
Y.,
Systematic review and meta-analysis of the clinical efficacy and adverse effects of Chinese herbal decoction for the treatment of gout. PLoS ONE.
2014;
9
(1)
:
e85008
.
View Article PubMed Google Scholar -
Minh-Hoa
T.T.,
J
J. Darmawan,
SL
S.L. Chen,
Hung
N.V,
Nhi
C.T.,
An
T.N.,
Prevalence of the rheumatic diseases in urban Vietnam: a WHO-ILAR COPCORD study. The Journal of rheumatology.
2003;
30
(10)
:
2252-2256
.
-
Hassan
F.I.,
Zezi
A.U. ,
Yaro
A.H.,
Danmalam
U.H.,
Analgesic, anti-inflammatory and antipyretic activities of the methanol leaf extract of Dalbergia saxatilis Hook.F in rats and mice. J Ethnopharmacol.
2015;
166
:
74-78
.
View Article PubMed Google Scholar -
Fei
Y.,
Ye
D.,
Fan
X.F.,
Dong
F.,
Effect of Dioscorea tokoro Makino extract on hyperuricemia in mice. Tropical Journal of Pharmaceutical Research.
2016;
15
:
1883-1887
.
View Article Google Scholar -
Ryu
K.H.,
Rhee
H.I.,
Kim
J.H.,
Yoo
H.,
Lee
B.Y.,
Um
K.A.,
Anti-inflammatory and analgesic activities of SKLJI, a highly purified and injectable herbal extract of Lonicera japonica. Biosci Biotechnol Biochem.
2010;;
74
(10)
:
2022-2028
.
View Article PubMed Google Scholar -
Kim
K.S.,
Choi
H.M.,
Yang
H.I.,
Yoo
M.C.,
WIN-34B May Have Analgesic and Anti-Inflammatory Effects by Reducing the Production of Pro-Inflammatory Mediators in Cells via Inhibition of IκB Signaling Pathways. Biomol Ther (Seoul).
2012;
20
(1)
:
50-56
.
View Article PubMed Google Scholar -
Kang
M.,
Jung
I.,
Hur
J.,
Kim
S.H.,
Lee
J.H.,
Kang
J.Y.,
The analgesic and anti-inflammatory effect of WIN-34B, a new herbal formula for osteoarthritis composed of Lonicera japonica Thunb and Anemarrhena asphodeloides BUNGE in vivo. J Ethnopharmacol.
2010;
131
(2)
:
485-496
.
View Article PubMed Google Scholar -
He
D.Y.,
Dai
S.M.,
Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall, a traditional chinese herbal medicine. . Frontiers in Pharmacology.
2011;
2
:
10
.
View Article Google Scholar -
Yang
Q.,
Wang
Q.,
Deng
W.,
Sun
C.,
Wei
Q.,
Adu-Frimpong
M.,
Anti-hyperuricemic and anti-gouty arthritis activities of polysaccharide purified from Lonicera japonica in model rats. Int J Biol Macromol.
2019;
123
:
801-809
.
View Article PubMed Google Scholar
Comments
Downloads
Article Details
Volume & Issue : Vol 7 No 5 (2020)
Page No.: 3760-3767
Published on: 2020-05-25
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 5903 times
- Download PDF downloaded - 1852 times
- View Article downloaded - 0 times
 Biomedpress
Biomedpress

