Autologous Bone Marrow Stem Cells combined with Allograft Cancellous Bone in Treatment of Nonunion
Abstract
Autologous cancellous bone graft is currently used as a gold standard method for treatment of bone nonunion. However, there is a limit to the amount of autologous cancellous bone that can be harvested and the donor site morbidity presents a major disadvantage to autologous bone grafting. Embedding viable cells within biological scaffolds appears to be extremely promising. The purpose of this study was to assess the outcome of autologous bone marrow stem cells combined with a cancellous bone allograft as compared to an autologous bone graft in the treatment of bone nonunion. Bone marrow aspiration concentrate (BMAC) was previously produced from bone marrow aspirate via a density gradient centrifugation. Autologous cancellous bone was harvested in 9 patients and applied to the nonunion site. In 18 patients of the clinical trial group after the debridement, the bone gaps were filled with a composite of BMAC and allograft cancellous bone chips (BMAC-ACB). Bone consolidation was obtained in 88.9 %, and the mean interval between the cell transplantation and union was 4.6 ± 1.5 months in the autograft group. Bone union rate was 94.4 % in group of composite BMAC-ACB implantation. The time to union in BMAC-ACB grafting group was 3.3 ± 0.90 months, and led to faster healing when compared to the autograft. A mean concentration of autologous progenitor cells was found to be 2.43 ± 1.03 (x106) CD34+ cells/ml, and a mean viability of CD34+ cells was 97.97 ± 1.47 (%). This study shows that the implantation of BMAC has presented the efficacy for treatment of nonunion and may contribute an available alternative to autologous cancellous bone graft. But large clinical application of BM-MSCs requires a more appropriate and profound scientific investigations.
Introduction
It is estimated that a small amount of bone fractures gives alterations in the consolidation process and lead to nonunion. This still happens even with good understanding of recent advancements in the biotechnology of bone healing Bastos Filho et al., 2012Giannotti et al., 2013aLiebergall et al., 2013. The surgical approach is still the most important tool in the management of nonunion: revitalizing, opening of the intramedullary canal, synthesizing, and filling the gap of the bone defect with autologous cancellous bone Giannotti et al., 2013aTressler et al., 2011. Autologous cancellous bone graft, which is the most often removed from the iliac crest, is currently used as the gold standard option. This is due to its high potentials of osteogenesis, osteoinduction and osteoconduction Bastos Filho et al., 2012Jäger et al., 2011. However, there is a limit to the amount of autologous cancellous bone that can be harvested in the reconstruction of extensive bone defects Yoshikawa et al., 2011. In addition, the donor site morbidity presents a major disadvantage to autologous bone grafting Jäger et al., 2011.
Various alternative treatment modalities to stimulate bone healing, including tissue engineering, have been investigated all with the aim of minimizing patient morbidity Dimitriou et al., 2011Fox and Genever, 2014Gómez-Barrena et al., 2015Kim and Cho, 2013. Embedding viable cells within the biological scaffolds appears to be extremely promising since it allows osteocompetent cells to generate new bone tissue and contribute to the improved tissue healing Connolly et al., 1989Gamie et al., 2012Giannotti et al., 2013a. Mesenchymal stem cells (MSCs) represent a good candidate cell source because of their biological characteristics and potential role for clinical bone regeneration Chanda et al., 2010Jäger et al., 2011Kuroda et al., 2014.
The purpose of this clinical study was to assess the outcome of autologous bone marrow mesenchymal stem cells combined with a cancellous bone allograft as compared to an autologous bone graft in the treatment of bone nonunion.
Materials and methods
Patient recruitment
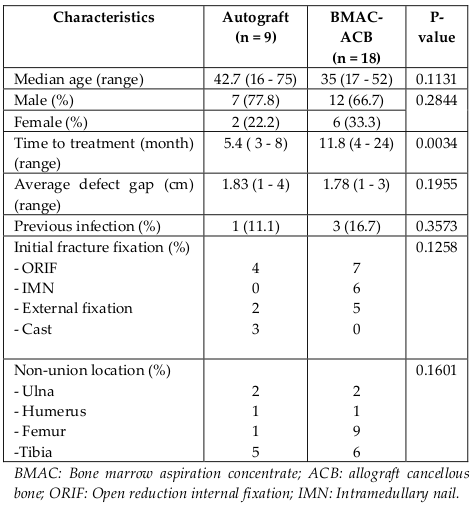
This prospective study was performed in patients who were treated at our Center from January 2013 to December 2014 with diagnosis of nonunion of a long bone fracture. The protocol for this clinical study was approved by the Committee of Medical Learning and Research Center. In total, 27 patients with nonunion were enrolled in this clinical trial. Autologous cancellous bone graft from the iliac crest was harvested in 9 patients in the control group and applied to the nonunion site. In 18 patients of the clinical trial group after the debridement, the bone gaps were filled with a composite of bone marrow MSCs and allograft cancellous bone chips. Bone fractures nonunion included for evaluation were femur, tibia, humerus, and ulna fractures. All the patients were presented with extraarticular fractures and Müller AO fracture classification. Patient demographic, medical history, initial fracture treatment, subsequent nonunion treatment, and operative and follow-up data were recorded.
A diagnosis of nonunion was made by the clinical examination and radiographic data. Clinical evidence of a nonunion was determined by documented pain and motion at the fracture site. "Nonunion" was determined by a lack of radiographic evidence of bone bridging on 3 of 4 cortices in two planes of χ-rays at 6 months after injury or a fracture that had not shown in any progression of healing over a three-month period Tressler et al., 2011. Clinical assessment and laboratories tests such as white blood cell count, erythrocyte sedimentation rate, and C-active protein were done to rule out infection. No patient was included in analysis if they were actively being treated for an infection at the fracture site at the time of nonunion revision. Patients were also excluded if purulence was visible at the nonunion site and intraoperative cultures demonstrated an infection Tressler et al., 2011. Exclusion criteria were ongoing treatment with immunosuppressant drugs including glucocorticosteroids, chemotherapy or colchicines. Patients were excluded if they were pregnant or during lactation. Patients with autoimmune deficiency syndrome, hepatitis, or a medical history of alcohol or drug abuse were also excluded Hernigou and Schuind, 2013Jäger et al., 2011.
Isolation and osteogenic pre-induction of MSCs
Bone marrow aspirates (300 -350 ml) were obtained by the Jamshidi vacuum. Both the posterior iliac crests of the patients were harvested under loco-regional anesthesia. The samples were collected at the Department of Hematology. Bone marrow mesenchymal stem cells (BM-MSCs) cultures were established as previously described Giannotti et al., 2013bHernigou et al., 2005a. Bone marrow aspiration concentrate (BMAC) was produced via a density gradient centrifugation using the SorvallTMcentrifuge (Thermo Scientific, MA, USA) at 3,670 rpm for 7 min. Afterwards, a total volume of 8 ml BMAC was mixed with freeze-dried allograft cancellous bone chips (ACB). To allow cellular adherence, the BMAC was incubated for 15 minutes with ACB as a composite of BMAC-ACB prior to transplantation ( Figure 1 ).
Surgical technique
The surgical procedure involved the correction and optimization of the fixation device, which had been used in the previously failed procedures. Subperiosteal decortication of the fragments was carried out. All the fibrous tissue at the fracture site was debrided until healthy bone was obtained. Sites of nonunion were completely excised and the medullary canal was opened. The bone edges were completely cleaned and revitalized. Then new bone stabilization procedures were performed using locking plates, interlocking intramedullary nails or an external device.
In case where autologous bone grafts were used for filling the patient’s bone defects, cancellous bone from the posterior iliac crest was harvested. In the clinical trial group, bone gap at the nonunion site was filled with a composition of BMAC-ACB. Each patient was treated with only one treatment modality and exclusively a graft was used. No patient received a combination of various treatment methods. All patients were limited from taking NSAIDs for pain relief. Paracetamol IV was used for the first post-operative day. Postoperative complications and complaints were monitored. Radiographs were evaluated after the surgery.
Assessment of bone healing
All patients were monitored using the same protocol during the postoperative period. Patients were followed up in the outpatient clinic for 1 month, 3 months, 6 months, 9 months, 12 months, 18 months, and 24 months after the procedure. The physical examination assessed pain, sensation of stability, and ability to walk with or without crutches. χ-rays were taken in two standard planes (anteroposterior and lateral) at all visits. Radiographic evaluation was performed via χ-ray analysis. Assessment of new bone formation and remodelling was based on the modified Lane and Sandhu radiological scoring system. Three experts blindly assessed the radiological scores, which were the sum of the scores of bone formation and remodelling. The score for bone formation was defined as 0 (no new bone formation), 1 (<25% new bone formation), 2 (25–50% new bone formation), 3 (50–75% new bone formation), or 4 (>75% new bone formation). The score of union was 0 (full fracture line), 2 (partial fracture line), and 4 (absent fracture line). The remodeling score was 0 (no evidence of remodeling), 2 (remodeling of the intramedullary channel), and 4 (full remodeling of the cortex). The maximum points could be achieved was 12.
Fracture healing was assessed by lack of pain during weight-bearing and bridging of three out of four cortices in both anteroposterior and lateral radiographic views Liebergall et al., 2013. Bone union was established when both clinical and radiographic evidence were in agreement Tressler et al., 2011. Evaluation of the radiographs as part of the clinical follow-up was performed by the non-blinded surgeons. Every side effects resulting from the procedure were assessed and recorded. Partial weight bearing was only allowed after the appearance of the bone callus and with signs of stability upon physical examination. The defined clinical protocol established that, if the patient did not present signs of bone consolidation six months after the procedure, a second intervention would be indicated, a situation considered a treatment failure.
MSCs analysis in vitro
The bone marrow cells had been achieved after a density gradient centrifugation were analysed. The total mononuclear cells from bone marrow samples were counted with the use of a SysmexXS800i Haematology Analyser (Sysmex Europe GmbH, Norderstedt, Germany). Cells then were displayed by immunofluorescence staining with antibodies against CD34 (Becton, Dickinson and Company, New Jersey, United States) at a concentration of 10-15 x 105 cells/ml. CD34+ cells were counted and expressed as percentage of CD34+ cells and CD34+ cells/ml. For cell viability evaluation, the Trypan Blue dye was used to enter cells with compromised membranes, making them appear dark upon bright field imaging. The percentage of live cells was calculated independently with dead cells. Colonyforming unit (CFU) and fibroblast colony-forming units (CFU-F) were used as an indicator of cell activity. Colony assays using the medium of MethocultTM H4434 Classic (Stemcell Technologies Inc., Vancouver, Canada). Fibroblast colonies were determined using mediums of MesenCultTM MSC Basal and MesenCultTM Stimulatory Supplements (Stemcell Technologies Inc., Vancouver, Canada). Finally, BMAC sample was tested for sterility using BD BACTECTM 9050 Blood Culture System (Becton, Dickinson and Company, New Jersey, United States). The results were displayed after 5 days of culturing the bacteria.
Statistical analysis
Variables are reported as mean and standard deviation (SD) or as raw percentages where applicable. The nonparametric Mann - Whitney U test was used to identify the significance of the differences between groups. The bone union score between the two treatment groups was compared with use of the log-rank test, and the data are presented with Kaplan-Meier curves. The statistical significance level was set at a probability value of p< 0.05. SPSS statistical software (version 15.0; SPSS, Chicago, Illinois) was used.
Results
No significant differences were found between the two groups in demographic characteristics or fracture parameters following previous procedures at the baseline time point. But there was a statistically significant difference in the mean time to treatment between both groups. For the group of BMAC-ACB graft, it was 11.8 months (range, 4 - 24 months) and the autograft group it was 5.4 months (range, 3 - 8 months) (p = 0.0034). The baseline characteristics of each group are shown in Table 1 . None of the patients presented perioperative complications in both groups. Mean followup time was 15.5 ± 7.7 months.
In the group of autologous iliac bone graft, intramedullary nailing was performed in four patients, and revision by locking plating was done in four patients. No revision of the existing external fixation was performed in one patient. There were no postoperative complications. There was one nonunion after 2-year follow-up in the case of nailing at the femur that needed revision of autologous cancellous bone graft. Bone healing was obtained in eight (88.9%) patients. The mean interval between surgery and union was 4.6 ± 1.5 months (range 3 - 7 months).
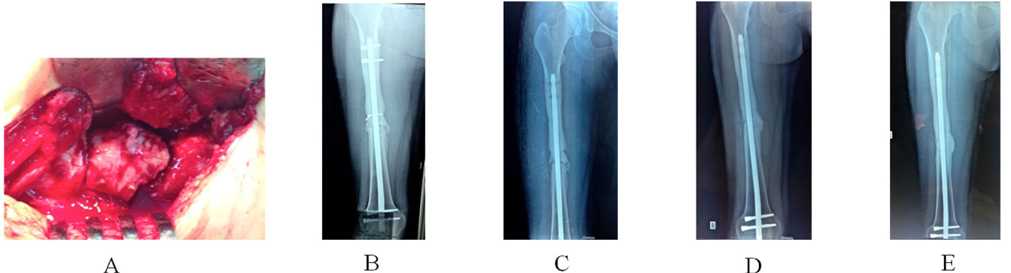
In the group of BMAC-ACB graft, revision by locking plating was done in nine patients. Nailing was performed in two patients. External fixation was carried out in one patient. No revision of the existing fixation was performed in six patients. There were two cases of postoperative wound infections. Antibiotic treatment was used during 6 weeks following the culture from the wound. Two patients were performed debridement, and one needed a local rotated flap to cover the wound (2 x 2 cm). These infections were controlled after two months of aggressive treatment. There was one patient that had a treatment failure due to nonunion and a broken nail after 12 months. Revision nailing and bone grafting were indicated for this patient. Bone healing after BMAC-ACB grafting was obtained in seventeen (94.4%) patients, where the interval between surgery and union was 3.3 ± 0.90 months (range, 2 - 5 months) in radiology. However, two patients showed delayed union of the fracture, manifested by no radiographic progress in any of the four cortices after 6 months. The first patient that had ongoing pain at the femoral fracture site was successfully treated by removal of the distal locking screws of the intramedullary nail and healing was evident at the 12- month follow-up ({fig3$A 22-year-old woman had a poly-trauma with splenic rupture associated IIIA Gustilo open fracture of thee left femur 7 months ago$Patient was needed a second debridement due to develop wound infection. Antibiotic treatment was used in 6 weeks following the culture from the wound. The infection was controlled after three months off aggressive treatment. (A) Clinical and laboratory examination were done to rule out infection. χ-ray was taken after external device removal and showed non-healing fracture of femur at 77 months after surgery. (B) χ-ray at one month after bone stability using interlocking intramedullary nail and a composite BMAC-ACB grafting. (C) Small new callus bridging the nonunion site on χ-ray at 3-month follow-up. (D) Bone consolidation was obtained by χ-ray at 6-month follow-up. (E) χ-ray showed mature remodeled callus in the area of the graft placement at 12-month follow-up.
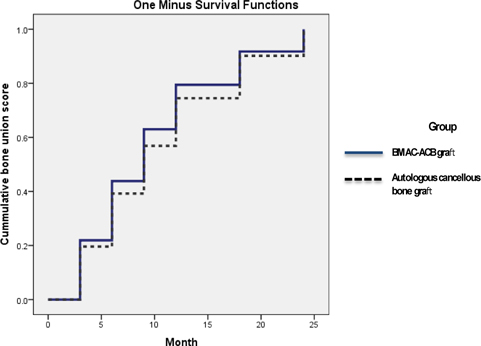
}). The second patient had no specific complaints and wanted a delay of a further intervention ( Figure 4 ). Patient started to have new small callus at the 8-monthfollow-up. No difference in bone healing rate was identified between the treatment groups (p = 0.4168), but there was a significant difference in the mean interval between surgery and union (p = 0.038). Survival analysis showed no significant difference in the time to bone healing between the two treatment groups during postoperative follow-up (logrank test) ( Figure 2 ).
In vitro data
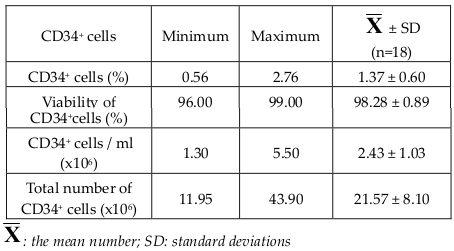
After concentration of bone marrow aspirates, progenitors prepared for transplantation contained a mean CD34+ cells ratio of 1.46 ± 0.74(%) (Range, 0.56 - 3.9%), a mean concentration of 2.43 ± 1.03 (x106) CD34+ cells/ml (Range, 0.13 - 5.5 x106 cells/ml), and a mean number of 21.57 ± 8.10 (x106) CD34+ cells (Range, 11.95 - 43.90 x106). Trypan Blue staining analysis showed a mean viability of CD34+ cells at 97.97±1.47(%) (Range, 92 - 99%) ( Table 2 ). Colonyforming unit (CFU) and fibroblast colony-forming units (CFU-F) were detected in all the samples incubatedin vitro. All samples of BMAC were cultured for bacteria, 100% bacterial cultures were negative.
Discussion
The present study shows that that application of BMAC-ACB in 17 out of 18 patients (94.4%) resulted in bone union during the 24-month follow-up period. The mean interval between surgery and union for BMAC-ACB implantation is 3.3 ± 0.90 months (range, 2 - 5 months), and lead to faster healing when compared to the autologous cancellous bone graft.
As is widely accepted, the "diamond concept" of bone fracture healing is applied to treating nonunion Fayaz et al., 2011Giannoudis et al., 2007Gómez-Barrena et al.,2015. A triangular-shaped complex of interactions between the potent osteogenic cell populations, the osteoinductive stimulus and the osteoconductive matrix scaffolds are extensively studied and applied in search for the optimal grafting material Giannoudis et al., 2007Khan et al., 2011Virk and Lieberman, 2012. Mechanical stability is added to the diamond model as a crucial factor for bone healing, and essential for the formation of a callus that bridges the fracture site allowing loads to be transmitted across the fracture line Fayaz et al., 2011Hernigou et al., 2005b.
Historically, autologous cancellous bone grafts, usually from the iliac crest, is the gold standard and represents the most common therapeutic approach in orthopaedics. It is the only graft capable of providing all 3 elements of bone regeneration including osteogenic and osteoinductive as well as osteoconductive properties Giannotti et al.,2013aHatzokos et al., 2011Tressler et al., 2011. However, a bone autograft is limited in quantity and its harvesting represents an additional surgical intervention and donor site morbidity Gómez-Barrena et al., 2015. Therefore, investigations are needed to provide a safe and effective alternative. Tissue engineering has appeared to be a very promising technique in several regenerative applications in different anatomic regions Fayaz et al., 2011Hernigou et al., 2006Jäger et al., 2011Pacini, 2014. Indeed, the combination of cells and scaffolds could represent the optimal solution for the management of many bone defects, accelerating fracture healing, and reducing complications Giannotti et al., 2013bIsmail et al., 2013Ohgushi, 2014. According to the above mentioned "diamond concept", BM-MSCs provide the most appropriate cells for inducing bone repair, as they have a strong osteogenic potential and are easily obtained by culturing iliac crest aspirates Akram et al., 2014Gómez-Barrena et al., 2015. But the graft of BM-MSCs should be seen as the catalyst for bone healing and not the only determining factor for the resolution of the disease. A surgical approach still remains the most important factor Giannotti et al., 2013b. BM-MSCs aspiration from the iliac crest contains osteoprogenitor cells and has osteogenic and osteoinductive but not osteoconductive properties Hatzokos et al., 2011. For the management of bone defects, autologous BM-MSCs have been applied together with scaffolds at the lesion site, exploiting their trophic and differentiative properties Hernigou et al., 2005aKuroda et al., 2014. The need for cell attachment, proliferation, and differentiation in tissue engineering requires the design of functional scaffolds, preferably bioabsorbable. Cancellous bone allograft offers a scaffold providing advantages in terms of biocompatibility and good osteoconductive and osteoinductive properties Giannotti et al., 2013b. There was no significant difference in the mean rate of union between the treatment groups, but there was the relatively short time to achieve union in the group of composite BMAC-ACB grafting as compared to the allograft group. The high rate of union and the relatively short time to union in the clinical trial group represent a good outcome of BM-MSCs implantation.
Previous studies have confirmed that it was important to increase the number of progenitor cells in the graft after implantation Connolly, 1998Hernigou et al., 2005a. Bone marrow aspirations combined with cell concentration techniques have been proposed to increase the density of progenitor cell populations Hatzokos et al., 2011Jacobsen et al., 2008. The importance of the concentration of cells may be related to the survival of progenitors. The amount of available oxygen is probably one of the most limiting factors after transplantation. Because the transplanted progenitor cells compete with other cells for oxygen, the limitation of the transplanted cells is the best way to optimize progenitor cell survival that contribute to the bone formation Hernigou et al.,2005bPountos et al., 2010. Hernigou et al. reported that autologous bone marrow must contain at least 1500 progenitor cells per milliliter in order to be effective for the treatment of nonunion Hernigou et al., 2005a. The mean ratio of CD34+ cells in bone marrow grafting prior injection in the study of Gangji et al. achieved 1.0 ± 0.2(%) of CD34+ cells, which are precursors of hematopoietic cells Gangji, 2004. According to the results of our studies, a mean CD34+ cells ratio was 1.46 ± 0.74(%), a mean concentration of autologous progenitor cells was 2.43 ± 1.03 x106 CD34+ cells/ml, and a mean viability of CD34+ cells was 97.97 ± 1.47(%). Bacterial cultures of BMAC were completely negative, and all the cultures of the CFU and CFU-F displayed positive staining in vitro. We believe that the application of BMAC is a safe procedure and that we can harvest a relevant amount of potent mesenchymal stem cells to let them differentiate into osteoblasts in vitro. Furthermore, the excellent bone regeneration by BMAC combined with ACB reduced the amount of autologous bone grafting procedures in our patient population and expedited the bone healing Jäger et al., 2011.
The disadvantages of these series are the limited number of patients, the diversity of indications, the various locations of nonunion, defect sizes, as well as a measurement of the number of CFU-F was not performed. Therefore, it is very difficult to allow conclusive judgments.
Conclusion
The result from this study, the implantation of BMAC has presented the efficiency in the treatment of bone nonunion in the initial outcome and may provide an available alternative to autologous cancellous bone graft. As the surgical procedure remains the treatment of choice for bone nonunion, tissue engineering using BM-MSCs could provide a useful method to accelerate and complete the bone union process. The use of BMAC-scaffold composites may contribute to improved outcome in patients. But large clinical application of BM-MSCs requiresmore appropriate and profound scientific investigations.
Abbbreviations
Mesenchymal stem cells (MSCs); allograft cancellous bone (ACB); bone marrow mesenchymal stem cells (BM-MSCs); bone marrow aspiration concentrate (BMAC); and fibroblast colony-forming unit (CFU-F).
References
-
M.
Akram,
M.
Irshad,
F.
Farooqi,
R.K.
Sah,
M.L.
Shahzad,
A.H.
Sarfraz,
S.M.
Awais.
Role of injecting bone marrow aspiration injection in treating delayed union and nonunion. JPMA The Journal of the Pakistan Medical Association.
2014;
64
:
S154-158
.
-
R.
Bastos Filho,
S.
Lermontov,
R.
Borojevic,
P.C.
Schott,
V.S.
Gameiro,
J.M.
Granjeiro.
Cell therapy of pseudarthrosis. Acta ortopedica brasileira.
2012;
20
:
270-273
.
-
D.
Chanda,
S.
Kumar,
S.
Ponnazhagan.
Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in diseases of the skeleton. Journal of cellular biochemistry.
2010;
111
:
249-257
.
-
J.F.
Connolly.
Clinical use of marrow osteoprogenitor cells to stimulate osteogenesis. Clinical orthopaedics and related research.
1998;
355
:
S257-S266
.
-
J.F.
Connolly,
R.
Guse,
J.
Tiedeman,
R.
Dehne.
Autologous marrow injection for delayed unions of the tibia: a preliminary report. Journal of orthopaedic trauma.
1989;
3
:
276-282
.
-
R.
Dimitriou,
E.
Jones,
D.
McGonagle,
P.V.
Giannoudis.
Bone regeneration: current concepts and future directions. BMC medicine.
2011;
9
:
66
.
-
H.C.
Fayaz,
P.V.
Giannoudis,
M.S.
Vrahas,
R.M.
Smith,
C.
Moran,
H.C.
Pape,
C.
Krettek,
J.B.
Jupiter.
The role of stem cells in fracture healing and nonunion. International orthopaedics.
2011;
35
:
1587-1597
.
-
J.M.
Fox,
P.G.
Genever.
Use of Adult Stem Cells for Orthopedic Regenerative Medicine Applications. Cell & Tissue Transplantation & Therapy.
2014;
6
:
19
.
-
Z.
Gamie,
G.T.
Tran,
G.
Vyzas,
N.
Korres,
M.
Heliotis,
A.
Mantalaris,
E.
Tsiridis.
Stem cells combined with bone graft substitutes in skeletal tissue engineering. Expert opinion on biological therapy.
2012;
12
:
713-729
.
-
V.
Gangji,
J.P.
Hauzeur,
C.
Matos,
V.D.
Maertelaer,
M.
Toungouz,
M.
Lambermont.
Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. J Bone Joint Surg Am.
2004;
86
:
1153-1160
.
-
S.
Giannotti,
V.
Bottai,
M.
Ghilardi,
G.
Dell'osso,
R.
Fazzi,
L.
Trombi,
M.
Petrini,
G.
Guido.
Treatment of pseudoarthrosis of the upper limb using expanded mesenchymal stem cells: a pilot study. Eur Rev Med Pharmacol Sci.
2013a;
17
:
224-227
.
-
S.
Giannotti,
L.
Trombi,
V.
Bottai,
M.
Ghilardi,
D.
D’Alessandro,
S.
Danti,
G.
Dell’Osso,
G.
Guido,
M.
Petrini.
Use of autologous human mesenchymal stromal cell/fibrin clot constructs in upper limb non-unions: long-term assessment. PloSone.
2013b;
8
:
e73893
.
-
P.V.
Giannoudis,
T.A.
Einhorn,
D.
Marsh.
Fracture healing: the diamond concept. Injury.
2007;
38
:
S3-S6
.
-
E.
Gómez-Barrena,
P.
Rosset,
D.
Lozano,
J.
Stanovici,
C.
Ermthaller,
F.
Gerbhard.
Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone.
2015;
70
:
93-101
.
-
I.
Hatzokos,
S.I.
Stavridis,
E.
Iosifidou,
D.
Karataglis,
A.
Christodoulou.
Autologous bone marrow grafting combined with demineralized bone matrix improves consolidation of docking site after distraction osteogenesis. The Journal of Bone & Joint Surgery.
2011;
93
:
671-678
.
-
J.
Hernigou,
F.
Schuind.
Smoking as a predictor of negative outcome in diaphyseal fracture healing. International orthopaedics.
2013;
37
:
883-887
.
-
P.
Hernigou,
G.
Mathieu,
A.
Poignard,
O.
Manicom,
F.
Beaujean,
H.
Rouard.
Percutaneous autologous bone-marrow grafting for nonunions. The Journal of Bone & Joint Surgery.
2006;
88
:
322-327
.
-
P.
Hernigou,
A.
Poignard,
F.
Beaujean,
H.
Rouard.
Percutaneous autologous bone-marrow grafting for nonunions. The Journal of Bone & Joint Surgery.
2005a;
87
:
1430-1437
.
-
P.
Hernigou,
A.
Poignard,
O.
Manicom,
G.
Mathieu,
H.
Rouard.
The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. Journal of Bone & Joint.
2005b;
Surgery
:
British Volume 87, 896-902
.
-
H.
Ismail,
P.
Phedy,
E.
Kholinne,
Y.
Kusnadi,
L.
Sandhow,
M.
Merlina.
Existence of mesenchymal stem cells in sites of atrophic nonunion. Bone and Joint Research.
2013;
2
:
112-115
.
-
K.
Jacobsen,
K.
Szczepanowski,
L.A.
Al-Zube,
J.
Kim,
S.S.
Lin.
The role of intraoperative bone marrow aspirate stem cell concentration as a bone grafting technique. Techniques in Foot & Ankle Surgery.
2008;
7
:
84-89
.
-
M.
Jäger,
M.
Herten,
U.
Fochtmann,
J.
Fischer,
P.
Hernigou,
C.
Zilkens,
C.
Hendrich,
R.
Krauspe.
Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. Journal of Orthopaedic Research.
2011;
29
:
173-180
.
-
W.S.
Khan,
F.
Rayan,
B.S.
Dhinsa,
D.
Marsh.
An osteoconductive, osteoinductive, and osteogenic tissue-engineered product for trauma and orthopaedic surgery: how far are we?. Stem cells international 2012.
2011
.
-
N.
Kim,
S.-G.
Cho.
Clinical applications of mesenchymal stem cells. The Korean journal of internal medicine.
2013;
28
:
387-402
.
-
R.
Kuroda,
T.
Matsumoto,
T.
Niikura,
Y.
Kawakami,
T.
Fukui,
S.Y.
Lee,
Y.
Mifune,
S.
Kawamata,
M.
Fukushima,
T.
Asahara.
Local transplantation of granulocyte colony stimulating factor-mobilized CD34+ cells for patients with femoral and tibial nonunion: pilot clinical trial. Stem cells translational medicine.
2014;
3
:
128
.
-
M.
Liebergall,
J.
Schroeder,
R.
Mosheiff,
Z.
Gazit,
Z.
Yoram,
L.
Rasooly,
A.
Daskal,
A.
Khoury,
Y.
Weil,
S.
Beyth.
Stem cell-based therapy for prevention of delayed fracture union: A randomized and prospective preliminary study. Molecular Therapy.
2013;
21
:
1631-1638
.
-
H.
Ohgushi.
Osteogenically differentiated mesenchymal stem cells and ceramics for bone tissue engineering. Expert opinion on biological therapy.
2014;
14
:
197-208
.
-
S.
Pacini.
Deterministic and stochastic approaches in the clinical application of mesenchymal stromal cells (MSCs). Frontiers in cell and developmental biology.
2014;
2
.
-
I.
Pountos,
T.
Georgouli,
G.
Kontakis,
P.V.
Giannoudis.
Efficacy of minimally invasive techniques for enhancement of fracture healing: evidence today. International orthopaedics.
2010;
34
:
3-12
.
-
M.A.
Tressler,
J.E.
Richards,
D.
Sofianos,
F.K.
Comrie,
P.J.
Kregor,
W.T.
Obremskey.
Bone morphogenetic protein-2 compared to autologous iliac crest bone graft in the treatment of long bone nonunion. Orthopedics.
2011;
34
:
e877-e884
.
-
M.S.
Virk,
J.R.
Lieberman.
Biologic adjuvants for fracture healing. Arthritis research & therapy.
2012;
14
:
225
.
-
T.
Yoshikawa,
Y.
Ueda,
M.
Koizumi,
T.
Ohmura,
Y.
Tanaka.
Treatment of fracture non-union with tissueengineered bone grafts. Stem Cell Studies.
2011;
1
:
4
.
Comments
Downloads
Article Details
Volume & Issue : Vol 2 No 12 (2015)
Page No.: 409-417
Published on: 2015-12-20
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 6997 times
- Download PDF downloaded - 1820 times
- View Article downloaded - 12 times
 Biomedpress
Biomedpress