
Euphorbia hirta as a gold mine of high-value phytochemicals: A comprehensive review of its pharmacological activities and possible role against SARS-CoV-2
- Department of Chemistry, Madhyanchal Professional University, Ratibad, Bhopal-462044, M. P, India
- Department of Applied Science, Madhyanchal Professional University, Ratibad, Bhopal-462044, M. P, India
- Department of Botany, Madhyanchal Professional University, Ratibad, Bhopal-462044, M. P, India
Abstract
Euphorbia hirta is a common medicinal plant in folk and traditional medicine systems. This plant has shown promising effects against several human ailments and infectious diseases. Therefore, it is important to summarize the medicinal activities and value of Euphorbia hirta. The main intent of this literature review was to summarize the phytochemical content and pharmacological applications of Euphorbia hirta. The literature review about the pharmacology and phytochemistry of Euphorbia hirta was collected from different global platforms, such as Scopus, ERIC, PubMed, and Web of Science. E. hirta has a rich phytochemistry and exhibits remarkable activity against respiratory diseases, gastrointestinal disorders and venereal diseases. Different extracts of this plant have shown significant preclinical anticancer propensity against an array of different cancer cell lines. It acts as a highly active antiviral agent and has shown pronounced activity against coxsackievirus, human immunodeficiency virus, dengue virus, poliovirus and simian immunodeficiency virus. A clinical study showed its inhibitory responses against flu and fever in dengue patients. Most importantly, the plant possesses remarkable inhibitory action on ACE, which aids SARS-CoV-2 entry into host cells. The multidimensional role of Euphorbia hirta as a potential antiviral agent suggests its possible application to control COVID-19 along with modern and Western medicinal strategies. In conclusion, the literature review regarding Euphorbia hirta showed its strong pharmacological applications, such as antimicrobial, antimalarial, anti-asthmatic, antioxidant, antiviral and anticancer activities. Further in-depth research is necessary to monitor its role in the management of viral diseases, especially COVID-19.
Introduction
is frequently known as “Asthma plant” in English and “Dudhi” in Hindi. The plant is widely distributed throughout the globe, and in Asia, it is mainly found in Yemen, Oman, Palestine, Taiwan, Syria, Lebanon, India, Bhutan, Pakistan, Nepal, Myanmar, Thailand, Sri Lanka, Indonesia, Malaysia, Papua New Guinea and the Philippines1. The plant belongs to the genus Euphorbia family of Euphorbiaceae. The morphological features of include a slender stem with hair development and many branches arising from it from base to top. The plant is annual purple or reddish in color and attains a height of approximately 40 cm. The leaves of the plant grow oppositely and are elliptical-oblong to oblong-lanceolate in shape. The leaves measure up to 1 – 2.5 cm in length with green color on the top side and pale color on the bottom side. The fruits are three-celled, yellow, keeled capsules, hairy, 1 - 2 mm in diameter, containing four-sided, three brown, wrinkled, angular, seeds2, 3, 4, 5. The plant has long served humanity in the form of traditional and folk medicine. In addition to other species of the genus Euphorbia also show medicinal importance and are being used in traditional medicine. A milky juice comes out of all the species of Euphorbia upon breaking, and this juice is considered to be more/less toxic and hence was used on arrows for hunting purposes in old times6. is a high-value medicinal plant possessing significant antimalarial, antifungal, antifertility, antispasmodic, sedative, antiasthmatic, anthelmintic and antibacterial properties2. Additionally, the plant has been found to have significant anticancer effects against a variety of aggressive cancer cells.
This review aims to summarize the phytochemical compositions and pharmacological activities of and tries to bridge the possible role of in the management of COVID-19, a going on global pandemic. Respiratory tract exposure to the external environment leads to high communicability of the disease. SARS-CoV-2 patients differ in clinical symptoms some show evident symptoms, and some remain asymptotic7. Asymptomatic patients with SARS-CoV-2 viral loads are the most active transporters leading to the fast spread of the disease because these patients are not aware of the disease until advanced stages. The initial clinical symptoms involve chills, fever, fatigue, cough, diarrhea, shortness of breath and respiratory symptoms. The generation of potential vaccines or capable drugs against SARS-CoV-2 infection is the global emergency right now. Unfortunately, the development of vaccines or potential drugs may take a longer time. Therefore, intermediate treatment methodologies are needed to address this global health issue. The government of the Republic of China is currently emphasizing Traditional Chinese Medicine (TCM) in controlling SARS-CoV-2 infection8, 9. Several clinical trials have already been initiated to study the efficiency of TCM against SARS-CoV-2 infection. In certain cases, patients along with Western medicine were sidewise supplied with TCM. The results showed that TCM induced synergistic effects with Western medicine against SARS-CoV-210. Treatments with medicinal plants and herbs are mostly symptoms and sign based. Herbal medicines with potential efficacy against specified targets against viruses could be evaluated for their activity against SARS-CoV-2, reliant on signs and symptoms11, 12. The prime focus of this review was to summarize the phytochemical constituents and pharmacological and medicinal importance of along with assessing its possibility to be used against COVID-19.

. (Figure 1) is a small annual, branched herb that can grow to 70 cm in height, purple or reddish in color with copious amounts of latex, and covered with sprout hairs.
Leaves: The leaves are opposite, biculate and simple, the stipules are linear, the leaf blade is lanceolate, oblong serrate, long elliptic, tapering, 3 – 4 cm long and 1 – 1.4 cm wide, and its margin is smoothly serrated.
Flowers: The monoecious inflorescence, an axillary or terminal cluster of flowers, is known as a cyathium, in which several cyathia are arranged in a cyme. The male and female flowers are in a pod and both appellation. The flowers are unisexual, male flowers are sessile, prophylls are linear, fringed, perianth absent and have a stamen, female flowers have a small peduncle, the perianth is fringed, the ovary is covered with tiny hairs above, 3-celled, has 3 - Styles, small and the tip is double. The flowering period is usually year round.
Fruit: The fruit is allomorphic, pistillate, elongated, 3-lobed, obtuse base covered with shoot hairs.
Seeds: Seeds are oblong, 4-sided prismatic, wrinkled and brownish pink in color, capsule 3-seeded, green and covered with fleshy spines, seeds smooth, hard mottled crustal skin with a white caruncle at the top enclosing oily endosperm13, 14, 15, 16, 17, 18, 19.
Roots: The root is a distinct and developed primary root (taproot system).
Classification: . belongs to the Euphorbiaceae family, known as the Spurge family. It is the largest family, consisting of almost 300 genera and 5000 species. Euphorbia is the largest genus of the Euphorbiaceae family and includes approximately 1600 species.
|
Kingdom |
Plantae |
|
Division |
Spermatomatophyta |
|
Class |
Dicotyledonae |
|
Order |
Euphorbiales |
|
Family |
Euphorbiaceae |
|
Genus |
Euphorbia |
|
Species |
Hirta |
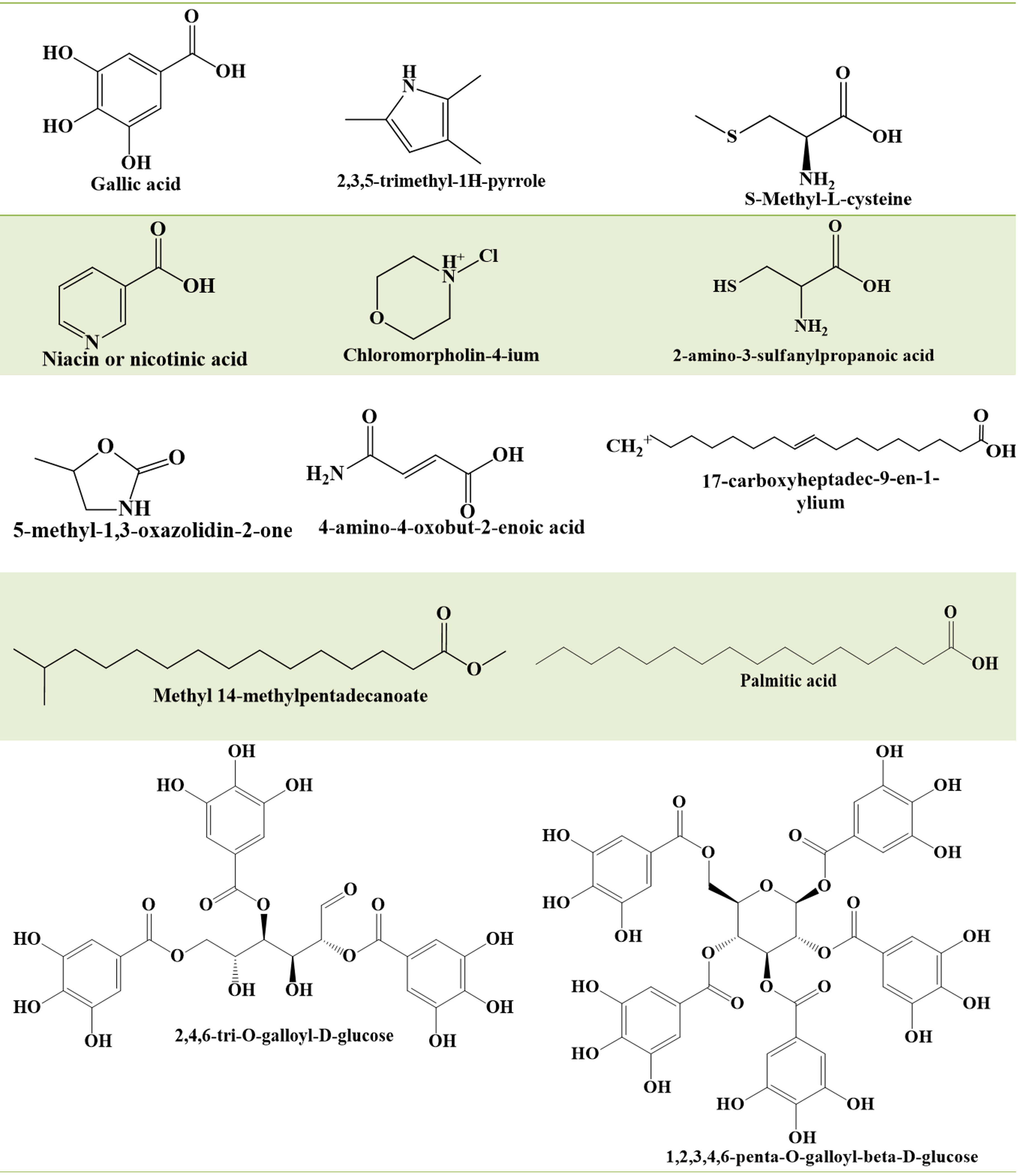
Structure of some of the phytochemical constituents from

Euphorbins (A-D) from
Bioactive secondary metabolites from
Plants are a source of highly active biological principles, making them helpful to humanity in regard to tackling key issues, including health20, 21. The plant bears a wide variety of phytochemicals, including reducing sugars, alkaloids, terpenoids, flavonoids, tannins, steroids, fats, proteins, gums, oils, mucilage, saponins, glycosides, cardiac glycosides, coumarins, anthraquinones and phenolic compounds22. Some of the important phytochemical constituents are summarized in Figure 2. The methanolic extract of has been identified with ten compounds, including palmitic acid, chloromorpholin-4-ium, S-methyl-L-cysteine, nicotinic acid, methyl 14-methylpentadecanoate, 2,3,5-trimethyl-1 H-pyrrole, 5-methyl-1,3-oxazolidin-2-one, 2-amino-3-sulfanylpropanoic acid, 17-carboxyheptadec-9-en-1-ylium and 4-amino-4-oxobut-2-enoic acid23. Six compounds were identified and isolated from leaves: 3,4-di--galloylquinic acid, gallic acid, myricitriu, quercitrin, 1,2,3,4,6-penta--galloyl-beta--glucose and 2,4,6-tri-O-galloyl-D-glucose24. Aerial parts of the plant were identified with quercitrin, afzelin, 1,3,4,6-tetra-O-galloyl-β-d-glucose, 2,4,6-tri-O-galloyl-β-d-glucose, euphorbins A-D (Figure 3), myricitrin, kaempferol, rutin, quercetin, gallic acid, and protocatechuic acid25. Furthermore, 11α,12α-oxidotaraxerol, α-amyrin, β-amyrin, taraxerone, β-amyrin acetate, taxerol, tannins and taraxerone have been reported from plants. Moreover, β-sitosterol, α-amyrin, 24-methylencycloartenol, camphol, leucocyanidol, euphorbianin and euphorbins A-E have also been isolated from the plant.
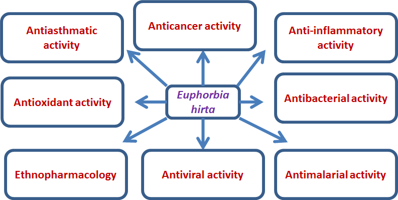
Biological activity profile of
Medicinal property of
Different parts of have shown numerous pharmacological and biological properties. The high biological value of the plant is primarily attributed to its high diversity in phytochemical content. Some of the biological activities are represented in Figure 4.
Ethnopharmacology
has a very high medicinal value. Ethnopharmacologically, is used to cure respiratory and bronchial disorders (hay fever, bronchitis and asthma), conjunctivitis and gastrointestinal diseases such as intestinal parasitosis, dysentery and diarrhea. Furthermore, shows significant tonic and hypotensive properties26. Stem sap of is used to cure eyelid styes caused by bacterial infection, and leaves are used against boils and swellings by making their poultice. The plant as a whole is used by humans against different diseases, such as fresh herb decoction in the treatment of thrush by gargling, dry decoction to cure skin disorders and decoction of roots, which is implemented in snake bites and for milk production in nursing mothers27. Antispasmodic and antiamoebic activities have been shown for polyphenolic extracts of the plant28, 29. Furthermore, the -isolated compound quercitrin has been reported to have remarkable antidiarrheal potential30, 31. It shows reflexive effects on cardiovascular systems in humans, such as the respiratory system32. The plant alcoholic extracts show tranquilizing effects on the genitor-urinary tract and report hypoglycemic effects in rats33, 34. The isolated compounds and solvent extracts of demonstrated substantial anticancer activities. extracts exhibit inhibitory effects on prostaglandin release, including D2, E2 and I2. It has also been reported to produce protective effects against contamination caused by aflatoxin in mustard, rice, maize and wheat crops35. Methanolic extracts of leaves have been shown to have strong antibacterial and antifungal properties. Itchy soles are treated by pounding, warming and rubbing the leaves of with coconut oil and turmeric. Plant latex is used to cure eye sores by applying it to the surma on the lower eyelids. A number of the ethnopharmacological uses of are listed in
Pharmacological activities of
|
No |
Activity |
Action |
|---|---|---|
|
1 |
Anti-allergic activity |
The ethanolic fraction of |
|
2 |
Antibacterial activity |
Different solvent extracts from |
|
3 |
Anti-diabetic activity |
The solvent extracts of stem, flower and leaf of |
|
4 |
Anti-diarrheal activity |
The leaves of |
|
5 |
Antioxidant activity |
Antioxidant activity of |
|
6 |
Antitumor activity |
Research has shown that the methanolic extract of |
|
7 |
Anxiolytic and sedative activity |
The antagonists of the GABAA receptor-benzodiazepine receptor-Cl channel complex with |
|
8 |
Diuretic activity |
The |
Antibacterial activity
Ethanolic extraction from leaves has been tested for its antibacterial activities against and . The extract showed strong inhibition of all these bacteria except . The minimum inhibitory values were calculated to be 74.61, 57.64, 22.55 and 54.09 mg/ml for and , respectively36. Unlike solvent extractions from the stem, bud and leaves of , their antimicrobial effects against were evaluated using the disc-diffusion method. The methanolic extract from leaves and bud exhibited very strong activity against with a zone of inhibition score of 20 mm and a zone of inhibition area score of 471.00 mm37. Chloroform and aqueous fractions of L. leaves have been reported to possess noncytotoxic but antibacterial effects against 38.
Anti-inflammatory activity
The medicinal herb has been reported to have remarkable anti-inflammatory effects. In a study, the aqueous and ethanolic extracts of were evaluated for their anti-inflammatory activity against carrageenan-induced inflammation in rats. It has been shown that both extracts produced substantial anti-inflammatory effects against the reference drug diclofenac sodium (50 mg/kg)39. In a similar study, the lyophilized aqueous extract from has been reported to suppress inflammation in carrageenan-induced rats starting from the concentrations of 100 mg/kg of body weight40. Furthermore, -hexane extracts from have been shown to inhibit inflammation in mouse models of phorbol acetate-induced ear inflammation41. The anti-inflammatory effects were found to be concentration-dependent. In another study, fractionated aqueous extract showed anti‑inflammatory activity on rabbit synovial fibroblasts42.
Antioxidant activity
possesses strong antioxidant activities in both animal models and . It has been shown to have strong free radical scavenging potency in various experimental models using hydroxyl radical scavenging, ABTS, and DPPH assays. The free-radical scavenging ability of the methanolic extract of was investigated. The results reported that the methanolic fraction of leaf extract produced a tremendous DPPH inhibition of 71.96±0.78%. The increasing order of DPPH scavenging activity of was stems (44.42±0.94%) < roots (48.59±0.97%) < flowers (52.45±0.66%) < leaves. The IC values calculated for stems, roots, flowers and leaves were 1.358, 0.989, 0.972 and 0.803 mg/mL, respectively43. Another study carried out by S. Asha and coworkers reported significant antioxidant activity for . They showed antioxidant activities for three types of extractions from through superoxide, DPPH and hydroxyl radical scavenging assays. Out of the three extracts (ethanolic, methanolic and aqueous), the ethanolic extract exhibited the highest antioxidant propensity with a significant IC value compared to the methanolic and aqueous extracts. Furthermore, a significant relationship was obtained between the phenolic content of the extracts and antioxidant activity, and the ethanolic extract showed a high phenolic content44. showed maximum free radical scavenging and antioxidant activities at approximately 0.25 mg/ml.
Anticancer activity
Several traditionally used medicinal plants are thought to have preventive effects against different human malignancies, including cancer. These plants are rich in chemical contents that show modulatory effects on different physiological functions and target the proliferation of cancer cells. has been reported to produce significant anticancer effects against acute myeloid leukemia HL-60 cells45. Furthermore, extracts from have revealed anticancer effects against squamous cell carcinoma, Hep-2 and malignant melanoma46, 47. Shao-Ming Chi et al., 2012 isolated (1’R,5’R)-5-(5’carboxylmethyl-2’-oxocyclopentyl)-3Z-pentenyl acetate, a cyclopentanone derivative from . The ethanolic extract was examined for cytotoxicity against the K562 and A549 cell lines. The outcome of the study revealed weak cytotoxicity against A549 cells (15.02 ± 11.60%) and remained almost inactive against K562 cells48. Sandeep . in 2011 evaluated the antitumor properties of 49. Aerial parts of were extracted using different solvents, including chloroform, ethanol and petroleum ether, and showed positive results for the presence of alkaloids, tannins, saponins, and flavonoids. The ethanol and chloroform extracts were reported to maximize the mean survival and inhibit the growth of solid tumors in administered mice. This antitumor activity was attributed to the manifestation of flavonoids.
Antimalarial and anti-asthmatic activities
has been reported to contain a pool of active phytochemicals that raise the medicinal value of the plant. has been termed an “Asthma plant”. It shows depressant effects on the respiratory system and reflexive effects on brochial tubes32. Additionally, methanolic extraction from aerial parts of by bioassay-guided fractionation has been evaluated for antiparasitic activity against . The key chromatographic fraction has been reported to show over 90% inhibition at 5 µg/ml against 50.
List of some of the common medicinal and aromatic plants with potential antiviral properties
|
Family |
Plant Species |
Mode of Action |
Plant Part |
Origin |
|---|---|---|---|---|
|
Acanthaceae |
Andrographis paniculata |
Antiviral |
Leaves |
India, Sri Lanka |
|
Acanthaceae |
Strobilanthes cusia |
Inhibits HCoV-NL63 via tryptanthrin; anti-influenza virus activity; anti-inflammatory potential |
Leaves, Whole plant |
Tropical Asia, Madagascar |
|
Adoxaceae |
Sambucus nigra |
Antiviral activity against HIV, HSV, influenza, hepatitis, and coxsackievirus |
Whole plant |
Europe and North America |
|
Adoxaceae |
Viburnum opulus |
Immunomodulation; anti-inflammatory effects |
Fruits |
Western and eastern, Siberia Eastern Europe, Caucasus, and Central Asia |
|
Alliaceae |
Allium sativum |
Inhibits avian coronavirus; antiviral, fungistatic |
Bulb |
Central Asia, Iran |
|
Anacardiaceae |
Rhus coriaria |
Antiviral potential |
Fruit |
Mild Mediterranean climates of western Asia and southern Europe |
|
Apiaceae |
Ferula assa-foetida |
Antiviral activity; great potency against H1N1; anti-inflammatory |
Oleo-Gum-resin |
Iran, Afghanistan |
|
Apiaceae |
Saposhnikovia divaricata |
High antiviral activity against PEDV corona-virus |
Whole plant |
China |
|
Apocynaceae |
Aspidosperma sp. |
Antiviral activity against avian metapneumovirus and other groups |
Whole plant |
South America |
|
Apocynaceae |
Gymnema sylvestre |
Inhibition of viral DNA synthesis; immunomodulation |
Leaves, Whole plant |
Asia, Africa, Australia |
|
Araliaceae |
Oplopanax elatus |
Immunomodulation and anti-inflammatory activities |
Whole plant |
North America, northeastern Asia |
|
Asteraceae |
Anthemis hyalina |
Inhibits coronavirus replication and expression of transient receptor potential gene family |
Whole plant |
Mediterranean region, south-west Asia to Iran |
|
Asteraceae |
Artemisia sp. (Artemisia absinthium) |
Reduces coronavirus replication; antibacterial, anti-inflammatory |
Whole plant |
Eurasia, north Africa, North America |
|
Asteraceae |
Cichorium intybus |
Immunomodulation; antiviral action against adenovirus type and 5HSV-1 |
Whole plant, Roots |
Eurasia, Mediterranean region |
|
Asteraceae |
Cynara scolymus |
ACE inhibitor, antiviral |
Flower heads |
Mediterranean region |
|
Asteraceae |
Echinacea angustifolia |
Antiviral activity against cold and flu viruses; inhibits discharge of pro-inflammatory cytokines and viral growth. |
Flowers |
North America |
|
Asteraceae |
Echinops sp. |
Antiviral, cough suppressant |
Trehala manna |
Iran |
|
Asteraceae |
Inula helenium |
Anti-inflammatory |
Rhizomes, Roots |
Caucasus, Eastern Europe, western Siberia, Central and Far East Asia |
|
Asteraceae |
Rhaponticum carthamoides |
Immunomodulation |
Roots |
Southern Siberia, Kazakhstan, Altay region |
|
Asteraceae |
Sphaeranthus indicus |
Antiviral activity against mouse coronavirus; anti-inflammatory and bronchodilation |
Whole plant |
Northern Australia, Indomalayan realm |
|
Bignoniaceae |
Arrabidaea samydoides |
Antiviral activity against HSV-1, vaccinia virus and murine encephalomyocarditis virus |
Whole plant |
South America |
|
Bignoniaceae |
Tabebuia sp. |
Antiviral potential |
Whole plant |
South America |
|
Boraginaceae |
Echium amoenum |
Antiviral |
Flowers |
Iran, Caucasus, Russia |
|
Brassicaceae |
Isatis tinctoria |
Inhibits cleavage activity of SARS-3CLpro enzyme; anti-inflammatory and strong antioxidant potential |
Roots extracts |
Caucasus, Central Asia, eastern Siberia, western Asia |
|
Cannabaceae |
Humulus lupulus |
Immunomodulation; antiviral activity against cold and influenza viruses, herpesvirus and hepatitis C; inhibition of virus replication |
Inflorescences |
North America, Europe, western Asia |
|
Crassulaceae |
Bryophyllum pinnatum |
Anti-inflammatory, immunomodulator |
Whole plant |
Madagascar |
|
Cupressaceae |
Juniperus communis |
Prevents replication, 3CLpro; antiseptic and anti-inflammatory |
Fruits |
Europe, North America, Asia |
|
Cupressaceae |
Thuja occidentalis |
Immunostimulation; antiviral activity against acute common cold |
Leaves Whole plant |
Upper northeastern, North and Central United States and Eastern Canada |
|
Elaeagnaceae |
Hippophae rhamnoides |
Anti-influenza activities and Immunomodulation |
Fruits |
Cold-temperate regions of Europe and Asia |
|
Euphorbiaceae |
Euphorbia sp. |
Antiviral activity against SIVmac251, HSV-2, HIV-1 and HIV-2 |
Roots |
North and South America, Southern Africa and Madagascar, Mediterranean region |
|
Fabaceae |
Acacia nilotica |
Inhibits HIV protease; cytotoxic and antiviral |
Whole plant |
Indian subcontinent, Middle East and Africa |
|
Fabaceae |
Alhagi maurorum |
Inhibits influenza and cold viruses; relieves cough, pectoral aches, fever, vomiting and thirst |
Gum tragacanth |
South-east Europe, south-west Asia |
|
Fabaceae |
Clitoria ternatea |
Antiviral |
Whole plant |
Indian subcontinent, Southeast Asia |
|
Fabaceae |
Desmodium canadense |
High antiviral activity toward coronaviruses |
Whole plant |
North America |
|
Fabaceae |
Glycyrrhiza glabra |
Immunomodulation; antiviral activity against human cytomegalo-virus, Epstein–Barr virus, HSV-1, and RNA viruses including H1N1, influenza A, and H5N1 |
Roots |
Mediterranean area, Iran-Turan, Azerbaijan |
|
Geraniaceae |
Pelargonium sidoides |
Decreases rhinovirus infection through regulation of binding viral proteins in bronchial cells. |
Leaves, Whole plant |
South Africa |
|
Hypericaceae |
Hypericum connatum |
High antiviral activity |
Whole plant |
North America, eastern Asia |
|
Lamiaceae |
Mentha piperita |
High antiviral activity against coronavirus group |
Whole plant |
Europe, Middle East |
|
Lamiaceae |
Mosla sp. |
Anti-influenza activity |
Whole plant |
Eastern and southeastern Asia, Himalayas |
|
Lamiaceae |
Ocimum kilimandscharicum |
Antiviral activity against HIV-1, SARS-CoV-2 |
Whole plant |
Central Africa, Southeast Asia |
|
Lamiaceae |
Origanum vulgare |
Respiratory and antiviral activity |
Leaves, Stems |
Mediterranean region, Southwestern and Western Eurasia |
|
Lamiaceae |
Rosmarinus officinalis |
Antiviral activity against human respiratory syncytial virus; immunomodu-lator; anti-inflammatory |
Whole plant |
Mediterranean region |
|
Lamiaceae |
Salvia officinalis |
High binding to COVID-19 proteases; Inhibits HSV-1 and SARS-CoV replication |
Whole plant |
Mediterranean basin |
|
Lamiaceae |
Scutellaria baicalensis |
Inhibit nsP13 by affecting the ATPase activity |
Roots |
China, Korea, Mongolia, Russian far east, Siberia |
|
Lamiaceae |
Stachys schtschegleevii |
Antiviral, anti-inflammatory and anti-SARS-CoV-2 |
Leaves |
Iran |
|
Lamiaceae |
Thymus vulgaris |
High antiviral activity toward coronaviruses; antioxidant effects |
Whole plant |
Southern Europe |
|
Lauraceae |
Cinnamomum cassia |
Antiviral, anti-inflammatory; inhibits attachment of human respiratory syncytial virus |
Bark |
Vietnam and eastern Himalayas, China |
|
Lythraceae |
Punica granatum |
Inhibits viral glycoproteins; antiviral action against influenza virus and HSV-1 |
Fruits, Peel, Seeds |
Iran to northern India, Mediterranean region |
|
Malvaceae |
Althaea officinalis |
Anti-inflammatory in diseases of the upper respiratory tract; antitussive, chest emollient, immuno-modulator, antiviral |
Whole plant |
Western palearctic, boreal area, Europe, Asia and Africa |
|
Malvaceae |
Firmiana simplex |
Immunomodulation; general tonic and adaptogenic drug |
Leaves |
South Japan, China and Indonesia |
|
Menispermaceae |
Stephania tetrandra |
Inhibits expression of HCoV-OC43 nucleocapsid and spike proteins; anticancer and immunomodulatory potential |
Roots |
China, Taiwan |
|
Plantaginaceae |
Plantago major |
Anti-inflammatory; antiviral activity against herpesviruses and adenoviruses |
Leaves, Whole plant |
Europe, Northern and central Asia |
|
Ranunculaceae |
Nigella sativa |
Antiviral activity against avian influenza virus (H9N2), Immunomodulator, broncho-dilator and anti-inflammatory agent |
Whole plant |
Eastern Mediterranean, northern Africa, Indian Subcontinent, western Asia |
|
Rhamnaceae |
Ampelozizyphus amazonicus |
Immunomodulation, anti-inflammatory |
Whole plant |
South America |
|
Rhamnaceae |
Ziziphus jujuba |
Antiviral activity; potential therapeutic agent for treating influenza |
Fruit |
Southeastern Europe to China |
|
Rosaceae |
Rubus sp. |
Antiviral effect against human influenza virus |
Fruits, Flowers |
Forest-steppe zones of Eurasia |
|
Rosaceae |
Rosa sp. |
Immunomodulatory effects; antiviral activity against HIV and HSV |
Completely matured fruits |
Europe, North America, Northwestern Africa |
|
Rutaceae |
Citrus trifoliata |
Antiviral against oseltamivir-resistant influenza virus |
Seeds |
Northern China and Korea |
|
Sapindaceae |
Litchi chinensis |
The plant inhibit SARS-3CLpro, while the isolated terpenoids suppress HIV-1 protease |
Seeds |
Southeastern China |
|
Saururaceae |
Houttuynia cordata |
Inhibits viral tRNA polymerase (RdRp) and SARS-3CLpro activity; activates IL-2 and IL-10 secretion |
Whole plant |
Southern Asia |
|
Solanaceae |
Hyoscyamus niger |
Viral inhibition; bronchodilator; antiviral effect against human influenza virus A/WSN/33 |
Whole plant |
Middle East, Asia, Continental Europe |
|
Theaceae |
Camellia japonica |
Strong inhibition of a member of coronavirus family that is porcine epidemic diarrhea virus through suppression of important protein and gene synthesis during replication |
Whole plant, Flowers |
East Asia |
|
Urticaceae |
Urtica dioica |
Inhibition of SARS coronavirus replication |
Leaves |
Europe, temperate Asia, and western North Africa |
|
Verbenaceae |
Vitex trifolia |
Strongly antiviral against and mouse coronavirus HSV, anti-inflammatory effects on lungs, immunomodulatory |
Whole plant |
French Polynesia, Tropical East Africa |
|
Zingiberaceae |
Zingiber officinale |
Inhibition of syncytial virus effecting human respiratory |
Rhizome |
Asia, Maritime Southeast |
|
Zosteraceae |
Zostera marina |
Strongly antiviral against influenza A virus |
Whole plant |
North America, Europe, Asia |
Antiviral activity of
Medicinal and aromatic plants have been a rich reserve for antiviral agents since time immemorial. Some of the medicinal plants with antiviral activity are listed in
In another study, the role of against dengue was demonstrated. Dengue disease is a viral disease caused by four distinct serotypic members of the family Flaviviridae and genus Flavivirus, including DENV 1-460. The plant has been regarded as a game changer in dengue management. Clinical investigation of has been recorded against age group 30 - 35, which after the supplementation revealed an approximately 70% reduction in flu-like symptoms caused by dengue61. The analysis of the ethanolic extract of the plant showed remarkable inhibition of plaque formation up to 85% and 34.7% against DENV-1 and DENV-262, respectively. Some of the studies that have been carried out for the calculation of the anti-dengue property of are listed in
List of some of the research investigations performed on the anti-dengue potential of
|
Study |
Results |
Experimental model |
Plant part(s)/extract |
|---|---|---|---|
|
Apostol |
The administration of |
Rats induced thrombocytopenic by ethanol (i.p injection) ( |
Decoction of fresh whole plant |
|
Arollado |
The consecutive treatment of rats with |
Rats induced thrombocytopenic by Anagrelide (i.p injection) ( |
Water extract of leaves |
|
Coloma |
A surveillance study of questionnaire was executed in Agoo, La Union, Philippines demonstrated Tawa-Tawa is very high in demand against dengue. The thrombocytopenic rabbits fed with |
Descriptive ethnobotanical survey. Aspirin-induced thrombocytopenia rabbits ( |
Expressed juice of Expressed juice of |
|
de Guzman |
The study showed very high percentage of women of 60-80 years of age have remarkable primary and secondary knowledge of using |
Ethnopharmacological survey |
Decoction of leaves or bark |
|
Mir |
Post 24 h of |
Clinical study, Sir Ganga Ram Hospital, Lahore using on admitted dengue patients. |
Herbal water |
|
Saptawati |
Virus inhibition by 34.7%. |
|
Ethanol extract of leaves |
|
Siva Ganesh |
Quercetin molecule has been reported to possess extraordinary binding efficacy against dengue virus. The leaves of |
Molecular docking study using phytochemicals with 2P40-methyl transferase, and 2FOM-dengue proteases of dengue |
Leaves |
|
Tayone |
Ethyl acetate fraction of dichloromethane and methanolic extracts of |
|
Ethyl acetate/methanol and ethyl acetate partitioning and tea of |
Furthermore, has been reported to have evident antiviral potency against herpes, coxsackie and polioviruses. has also been reported to have selective antiviral activity against HSV-1 with an MIC value of 0.1 mg/ml63. The leaf extract of plant has been reported to impart protective cover against potato virus X in both systemic and hypersensitive hosts. The active constituent actinomycin D was systemically sensitive toward the virus X64.
Possible role of against SARS-CoV-2
Unfortunately, communicability and COVID-19 infection are growing rapidly each day, causing huge human and economic losses globally65. The common clinical symptoms identified among COVID-19 patients include cough, shortness of breath, fever, and respiratory symptoms (such as inflammation caused by allergy to the pathogen). is an important medicinal plant involved in the global traditional medicine core, including Ayurvedic medicine and Traditional Chinese Medicine66. The lyophilized aqueous extract of revealed potential antipyretic, anti-inflammatory and analgesic actions in xenografted mice and rat models. The antipyretic effects of were evaluated via yeast-induced hyperthermia and showed potential activity at 100-400 mg/kg67. Furthermore, writhing and hot plate tests showed anti-analgesic activity in a dose-dependent manner at 20 mg/kg and 25 mg/kg, respectively. Additionally, strong anti-inflammatory activity of was observed in carrageenan-induced edema test rats at 100 mg/kg68. A clinical study in dengue patients performed by S. D Pareera at Sir Ganga Ram Hospital Lahore revealed that the administration of an aqueous extract of orally enhanced the total leucocyte count and platelets in patients aged 30-55 years. Moreover, 70% of the patients showed a response of lowering flu symptoms and fever. Moreover, the ethanolic extract of demonstrated significant inhibition of dengue virus stereotypes 1 and 269. is also known as an asthma plant and possesses remarkable activity against asthma70. Diarrhea is a key symptom commonly identified in COVID-19 patients. plant has been used against several gastrointestinal disorders, including diarrhea and ulcers. The methanolic extract of has been identified with rich flavonol glucoside content, including afzelin, myrcitrin and quercitin. The antimicrobial analysis of these compounds yielded IC values of 1.1, 5.4 and 4.1 against malarial parasites, respectively51. Furthermore, the plant has been reported to have high free radical scavenging properties. The maximum DPPH scavenging activity was reported by leaves, followed by flowers, roots and stems (72.96 ± 0.78%, 52.45 ± 0.66%, 48.59 ± 0.97%, and 44.42 ± 0.94%)71. Furthermore, has been reported to induce potential nonspecific immune responses, such as phagocytic ratio and lysozyme activity pathogen-infected fish model72. At higher concentrations, the plant was successful in eliminating Aeromonas hydrophila from the kidney and blood and enhanced the numbers of WBCs, RBCs and hemoglobin in test fish. Additionally, leaf extract enhanced the fabrication of log antibodies73. also showed potential immunomodulatory effects against animal models. The maximum inhibition was recorded at 100 and 200 mg/kg, wherein it remarkably blocks the generation of cell-mediated immune responses (IL-2, TNF-α, IFN-γ, CD3, CD4 and CD8)74.
SARS-CoV-2 attaches to the host cell using the receptor-binding domain (RBD) in its spike protein75. The RBD recognizes the ACE2 binding ridge on the outer cell membrane of the host cell, which leads to smooth entrance. The SARS-CoV-2 RBD bears an ACE2-binding ridge with a more compact conformation. Moreover, two virus-binding hotspots at the RBD–ACE2 interface are stabilized by several residue changes. The methanolic extract of roots and leaves has been reported to possess substantial angiotensin converting enzyme (ACE) anti-dipsogenic and inhibition activities. The extract suppressed the activity of ACE 50% at 160 μg and 90% at 500 μg76. The possible inhibition of the interface between SARS-CoV-2 and human host cells is shown in Figure 5.

Target site for possible inhibition of SARS-CoV-2 entrance to host human cells. Adapted from Brown
Future prospects
It is essential and needed of this era to continue the expansion of drug development and therapeutics based on plants and their chemical composition. Drugs and therapeutics based on plants are economical (cost effective) and are believed to be less toxic than synthetics. Cancer is a global health problem, making it difficult for scientists and researchers to overcome this ailment. Plants have assisted humanity against several malignancies in the past, and they are believed to do so currently and in the future as well. There are several drugs based on plants that have been approved for cancer chemotherapy, such as Taxol and paclitaxel. has a substantial potential to inhibit different cancers in humans due to its rich phytochemistry and active constituents. This plant bears a unique class of compounds called euphorbins, and they are complex in structure and active in nature. Therefore, it is believed to possess remarkable pharmacological potential, which needs to be explored.
It is highly recommended to push on progress in the field of potential antiviral therapeutics designed on natural products and their synthetic derivatives. Moreover, to look for therapeutics against coronaviruses, natural products have been the leading sources that have assisted human civilization in overcoming health hazards since the ages. The therapeutics designed on natural products have significant benefits over the synthetic ones, such as their cost effectiveness and miniscule or lack of side effects. Despite noteworthy developments in the field of vaccine development in this modern era, we lag behind in terms of developing breakthrough vaccines for several viruses, including SARS-CoV-2. Therefore, it seems to be a very difficult job to develop a potential treatment methodology for the management of such infectious viral diseases. However, plants such as and their bioactive phytochemicals have tremendous potential to serve humanity in overcoming these infectious diseases. Based on docking studies and the antiviral properties of phytochemicals, could also prove advantageous against coronaviruses. The rapid genomic mutations in SARS-CoV-2, HIV and HSV are the key drawbacks of antiviral therapeutics in targeting specific proteins and genes. The plant has huge potential against COVID-19, as it showed against different viruses, such as malaria, HSV and dengue. The plant has a strong antiviral property and has significant potential to target key sites, enzymes and replication of SARS-COV-2. Therefore, we recommend clinical investigations of against this lethal disease.
Conclusions
is a valuable medicinal plant used globally in different traditional systems of medicines. It has been reported to have various bioactivities against a wide array of human disorders. Most importantly, the plant as a whole bears a huge variety of chemical entities that enhance its therapeutic potential. The plant as a whole has been shown to have remarkable antiviral potential against HIV, DANV, HSV, etc. and enhancing immune responses against pathogens. It has great potency for free radical scavenging and ACE inhibition. Therefore, these features of may play an advantageous role throughout the management of highly infectious and deadly viral diseases such as COVID-19.
Abbreviations
SARS-CoV-2: Severe Acute Respiratory Syndrome Corona Virus-2, ACE: Angiotensin-converting enzyme, ABTS: 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid, CD: Cluster of differentiation, COVID-19: Corona Virus Disease 2019, DPPH: 2,2-diphenyl-1-picrylhydrazyl, DENV: Dengue Virus, HSV: Herpes Simplex Virus, HIV: Human Immunodeficiency Virus, IL-2: Interleukin-2, IFN-γ: Interferon gamma, MIC: Minimum inhibitory concentration, RBC: Red Blood cells, RBD: Receptor Binding Domain, WBC: White Blood Cells, TCM: Traditional Chinese Medicine, TNF-α: Tumor Necrosis Factor Alpha
Acknowledgments
Aadil Khursheed is highly thankful to Dr. Vikrant Jain and the whole Department of Chemistry, Madhyanchal Professional University, for providing academic guidance and platform.
Author’s contributions
All authors equally contributed to this work, read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

