Culture and differentiation of cytokine-induced killer cells from umbilical cord blood-derived mononuclear cells
Abstract
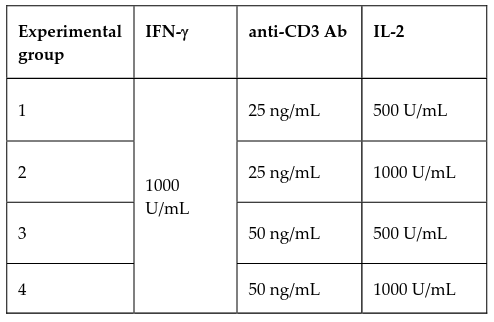
Cytokine-induced killer cells (CIK) are cytotoxic T cells, which have both NK and T cell properties. These cells are characterized by potent, non-MHC-restricted cytotoxicity and reduced alloreactivity, which make them appealing for use in adoptive immunotherapy of cancer and virus infections. In this study, CIK cells were generated by stimulating umbilical cord blood-derived mononuclear cells (UCB-MNCs) with interferon-gamma (IFN-g) on day 0. Anti-CD3 antibody and interleukin-2 (IL-2) were added after 24 hours at four different experimental concentration combinations in order to identify the optimal cytokine amounts for CIK cell proliferation. Cells were collected at four time points over a 21-day period (day 0, 7, 14, 21) for analysis of cell marker presentation using flow cytometry, as well as transcription-level cytokine production using RT-PCR. The results showed that in the 21-day culture, the average final expansion levels of CD3+CD56+ CIK cell were in the range of hundredfold, accounted for 26% in the bulk culture. Most important, these cells strongly expressed granzyme B (80.87%), a potent factor involved in cell-mediated cytotoxicity. These CIK cells also transcriptionally overexpressed the three cytokine genes that produce IFN-g, tumor necrosis factor-alpha (TNF-a), and IL-2; these are key for immune cell mobilization against tumors as well as foreign pathogens. Our research establishes an effective cytokine concentration and time protocol for use in generation of CIK cells from UCB-MNCs, potentiating greater applications of CIK cell-adoptive immunotherapy in both research and clinical settings. Thus, the 3rd and 4th experimental conditions both stimulated CIK cell differentiation with 50 ng/ml of anti-CD3 antibody, but with IL-2 concentrations of 500 U/ml and 1000 U/ml, respectively.
Introduction
Adoptive cellular immunotherapy (ACT) is the transfer of ex vivo-cultured immune cells primed for antitumor reactivity into a tumor-bearing host. It takes advantage of the host’sown ability to destroy tumors by stimulating immune effector cells to better recognize and eliminate tumor cells. Many different types of immune cells have been used in ACT, including natural killer (NK) cells, tumor-infiltrating lymphocytes (TILs), lymphokine-activated killer (LAK) cells, and cytokine-induced killer (CIK) cells. Among them, CIK cells appear to be the most promising cytotoxic effector cell type.
CIK cells are a heterogeneous subset of T lymphocytes with NK functional properties. The qualifier “cytokine- induced killer” indicates that they are generated via administration of cytokines during in vitro culture Arafar, 2014Linn and Hui, 2010. Cells which have the most potential effector function in CIK culture coexpress CD3 and CD56 surface molecules; possess a potent, MHC-unrestricted tumor-killing ability; and significantly reduced alloreactivity Sangiolo et al., 2008. CIK cells recognize tumor cells through the binding of CIK NKG2D receptors with tumor cell ligands such as MHC class I polypeptide-related sequences A and B (MICA and MICB); they then facilitate tumor cytolysis through secretion of perforin and granzyme degranulators Mehta et al.,1995Pievani et al., 2011Schmidt-Wolf et al., 1993. CIK cells display greatly reduced alloreactivity across the MHC barrier, theoretically due to abundant production of IFN- γBaker et al., 2001. Finally, CIK cells secrete IL-2, IL- 10, IL-6, IFN-γ, and TNF-α, which promote cytotoxic immune cell activity; and do not secrete suppressors such as IL-4, IL-7, and IL-12.
Initially described by Schmidt et al. and further characterized by Ortaldo et al., CIK cells are capable of killing a wide range of tumor cells, and hold great promise in the field of adoptive immunotherapy for cancer treatment Ortaldo et al., 1991Schmidt et al.,1986Schmidt-Wolf et al., 1991. These cells can be amplified in culture media from bone marrow, peripheral blood, or umbilical cord blood upon timedependent addition of IFN-γ, anti-CD3 antibody, and IL-2, as well as other cytokines. After 21 to 28 days of culture, CD3+CD56+ cell expansion ranges from one hundred to one thousand fold Hoyle et al., 1998Lu and Negrin, 1994Schmidt-Wolf et al., 1991. Other studies have also shown that functional CIK cells can be differentiated from the MNCs of either fresh Durrieu et al., 2013Introna et al., 2006Zhang et al., 2015 or banked Pham et al.,2015 umbilical cord blood, and effectively used in combination with other treatments for, among others, the relapse of acute leukemia after cord blood transplantation Introna et al., 2010, advanced solid malignancies after first-line chemotherapy failure Niu et al., 2011, and esophageal cancer Wang et al., 2014.
However, a standard protocol for umbilical cord blood-derived CIK cell generation has not been established. Previous studies have shown that CIK cells can be generated effectively from UCB-MNCs in different complete growth media, but vary with regards to cytokine concentrations of anti-CD3 antibody and IL-2 following 1000 U/mL IFN-γ Introna et al., 2006Introna et al., 2010Schmidt-Wolf et al., 1991Zhang et al., 2014Zhang et al., 2015. We split commonly used concentrations into four different experimental groups and used RPMI-1640 complete medium to create four disparate environments for stimulation of our CIK cells. These induced cells were then analyzed for cell marker expression and transcription-level cytokine production to determine the optimum cytokine concentrations for CIK cell generation.
Materials and Methods
Human materials
Cord blood samples were collected from three healthy parturitions at Van Hanh General Hospital (HCMC, Vietnam) following consent from donors and ethics committees.
Cells
Mononuclear cells (MNCs) were extracted from the buffy coat layer isolated from umbilical cord blood using Ficoll-Paque density gradient (GE Healthcare, Sweden) centrifugation at 400 g for 30 min at 25°C. The cells were then washed and resuspended at concentrations of 1-2 million (106) cells/mL in RPMI 1640 media containing 10% FBS and 1x Antibiotic-Antimycotic (Gibco, USA).
Normal human fibroblasts were thawed from the cell bank of the laboratory of Stem cell Research and Application. These cells were seeded into DMEM/F12 medium containing 10% FBS and 1x Antibiotic- Antimycotic (Gibco, USA) in a T25 flask.
Generation of CIK cells
To generate CIK cells, UBC-MNCs were cultured in RPMI complete medium (RPMI 1640 with 10% FBS and 1x Antibiotic-Antimycotic); 1000 U/mL IFN-γ (Santa Cruz, USA) was added to the medium at day 0. After 24 hours, anti-CD3 antibody (Santa Cruz, USA) and IL-2 (Santa Cruz, USA) were added to the culture in 4 different experimental concentrations as indicated in Table 1 to promote CIK cell proliferation. Cells were replenished with IL-2-containing fresh complete media every 2-3 days to maintain experimental concentrations.
Immunophenotype analysis by flow cytometry
Cells were harvested at four different time points (days 0, 7, 14, and 21) to analyze surface and intracellular markers by flow cytometry according to the manufacturer's protocol (BD Biosciences, USA). Briefly, fixed cells were stained in a covered ice bucket with the following: Tritest FITC-labeled anti-CD4 / PElabeled anti-CD8 / PerCP-labeled anti-CD3 antibodies (BD Biosciences, USA); APC-labeled anti-CD56 antibodies (Life Technologies, USA); and PE-labeled antigranzyme B antibodies (Life Technologies, USA) for 30 minutes each. For intracellular granzyme B staining, cells were permeabilized with FCM Permeabilization Buffer (Santa Cruz, USA) for 5 minutes at room temperature on a rotator prior to incubation with the antibodies. Unlabeled cells were used as controls in this experiment. All cells were analyzed using BD FACSCalibur (BD Biosciences, USA) and CellQuest Pro software with 10,000 events.
Cytokine production assay by RT-PCR
Total cellular RNA was extracted with the use of easy-BLUETM Total RNA Extraction Kit (iNtRON, Korea) from cells at days 0, 7, 14, and 21.
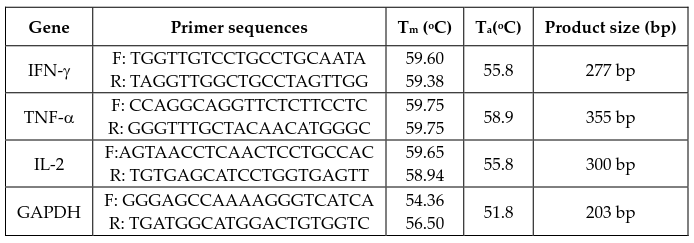
Cytokine gene expression was detected using a twostep RT-PCR. First, the complementary DNA was synthesized with qPCRBIO cDNA Synthesis Kit (PCR Biosystems, UK), according to the manufacturer’s protocol. PCR was then carried out in 10-μL final volumes using a Taq PCR Kit (BioLabs, USA), according to the manufacturer's protocol. The reaction was performed in a thermal cycler (Eppendorf, Germany) with amplification over 30 cycles at 94°C for 20 s (denaturing), 50-60°C for 10 s (annealing), and 72°C for 30 min (primer extension).
The primers (AIT Biotech, Singapore) used in this experiment are listed in Table 2 (F: forward, R: reverse). cDNA for GAPDH was co-amplified to assess the quality of the samples. The PCR products were electrophoresed in 1.2% agarose gels and stained with 0.5 μg/mL ethidium bromide. Band intensity was measured using ImageJ software (NIH) and for each group, the intensity of cytokine gene bands was normalized to that of the technical control (GAPDH).
Results
Generation of CIK cells
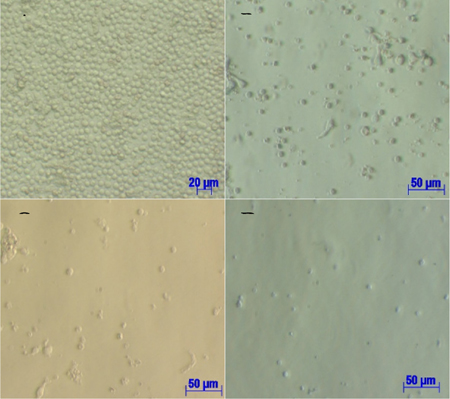
This study was carried out to determine the optimal cytokine concentrations for generation of CIK cells from UCB-MNCs using three experimental samples in four different concentration groups under a 21-day protocol. There were no differences in cell phenotypes among the four groups when observed under an inverted microscope (Olympus, Japan) ( Figure 1 ). On day 7, suspended cells were spherical in shape and cell sizes were uneven but generally smaller than the original MNC size ( Figure 1B ). On days 14 and 21, suspended cells were of equal sizes and generally smaller than cells from day 7 ( Figure 1C and Figure 1D ). By the end of the culture period (day 21), quantities of induced cells had begun to decrease due to cell death.
Phenotype analysis
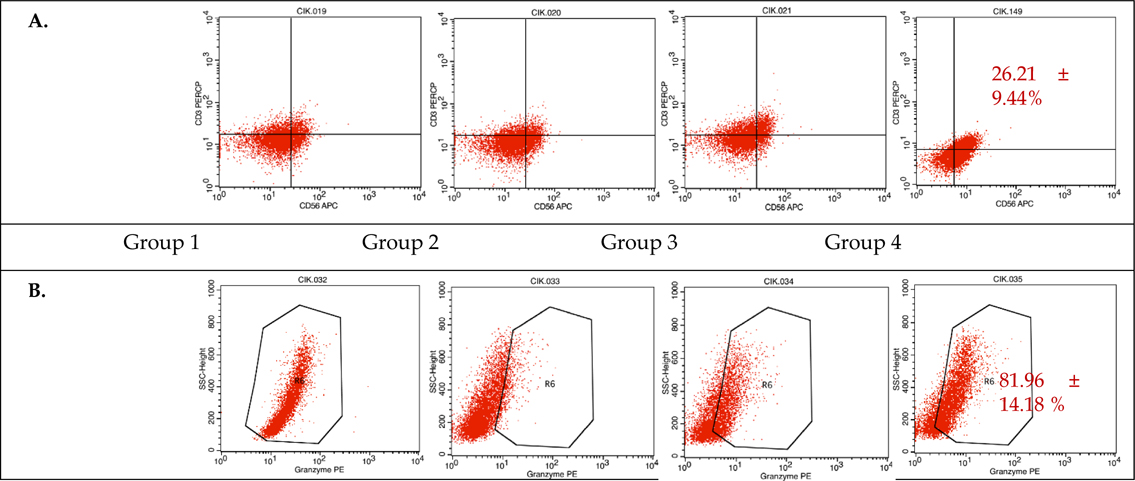
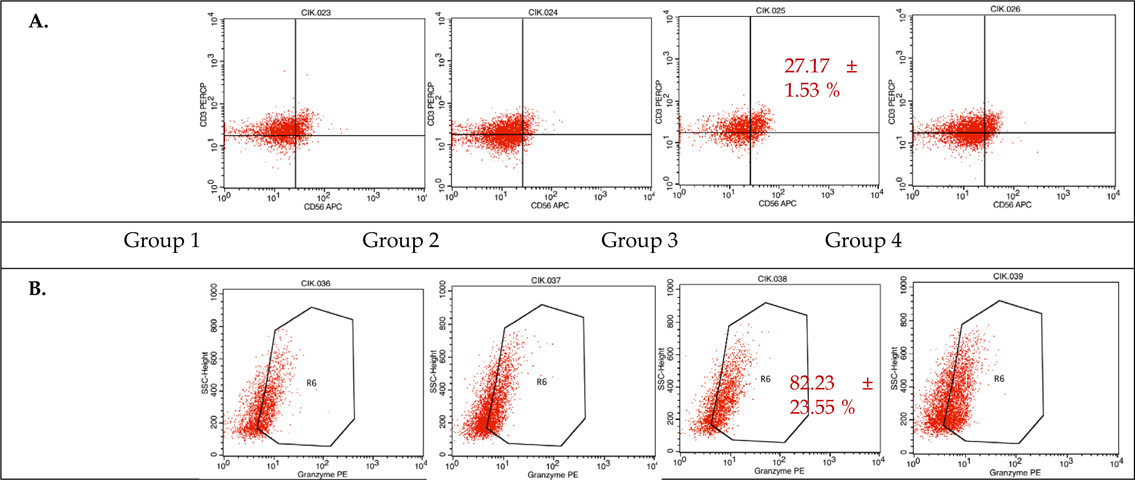
During CIK induction, cells were harvested at day 0, 7, 14, and 21 to analyze the presentation of cell markers CD3, CD4, and CD8 (data not shown), as well as CD56 and granzyme B by flow cytometry. The results of combined flow cytometry analyses of the three samples for each of the four experimental groups at day 14 and 21 are shown in Figure 2 and Figure 3 , respectively.
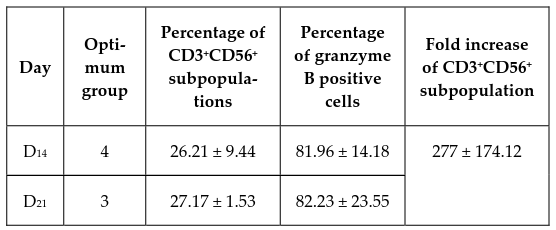
Analyzing at day 14, samples 1 and 2 from experimental group 4 (50 ng/mL anti-CD3 and 1000 U/mL IL-2) ( Figure 2 ) had the largest proportions of CD3+CD56+subpopulations, at 19.53% and 32.88%, respectively. The induced cells in samples 1, 2, and 3 from experimental group 4 also had the highest intracellular levels of granzyme B, at 72.73%, 74.86%, and 98.29%, respectively. These data indicate that the experimental group 4 protocol provides the best results after a 14- day culture period.
Immunophenotyping at day 21, however, showed that sample 2 from experimental group 3 (50 ng/mL anti- CD3 and 500 U/mL IL-2) had the largest CD3+CD56+subpopulation at 26.08% ( Figure 3 ). Samples 2 and 3 from group 3 also expressed the highest levels of granzyme B at 65.57% and 98.88%, respectively. These data indicate that the experimental group 3 protocol provides the best results after a 21-day culture period.
Proliferation of CIK cells
Table 3 summarizes the percentages of CD3+CD56+ subpopulations corresponding to their optimum protocols at day 14 and 21. In summary, there were about 20% to 33% CD3+CD56+ subpopulations at the end of both culture time points, with the final expansion rate of CD3+CD56+ cells in the range of 260 to 700 fold.
Cytokine production assay
In this experiment, normal human fibroblasts were used as a negative control for cytokine gene expression analysis, as fibroblasts produce very low levels of TNF-α and do not produce IFN-γ and IL-2 ( Figure 4 ). GAPDH was used as the internal control and showed a clear 205-bp band, indicating that the PCR reactions proceeded normally.
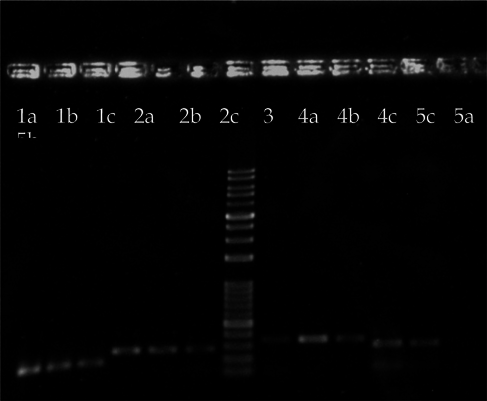
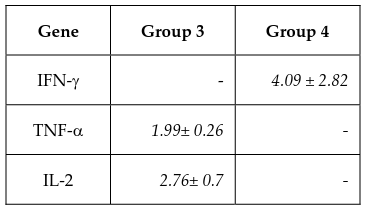
Cells were harvested to extract total RNA and perform RT-PCR at day 0, 7, 14, and 21. Results from the electrophoresis of the RT-PCR products of the CIK cell samples are shown in Figure 5 . Band intensity was measured and normalized to the GAPDH band of each sample, including the negative control, using the ImageJ software. The relative levels of CIK gene expression as compared to fibroblast samples were then determined ( Table 4 ).
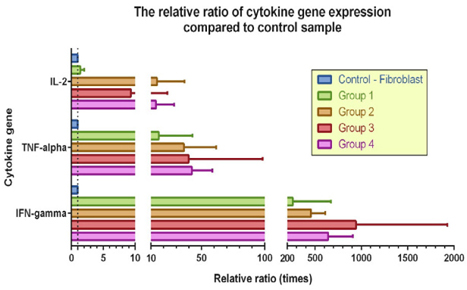
Figure 6 Figure 6 The relative ratio of cytokine gene expression normalized to their respective control samples Experimental protocols 3 and 4 yielded the best results.
According to the ImageJ analysis of the RT-PCR results, at the end of the culture, the induced cells in sample 1 from experimental group 4 and samples 2 and 3 from group 3 exhibited the highest expression levels of IFN-γ, TNF-α, and IL-2 genes ( Table 4 ), in accordance with the cell marker analyses performed using flow cytometry.
Discussion
CIKs are a heterogeneous population of cells displaying a mixed T-NK phenotype, non-MHC-restricted tumor-killing activity, and low alloreactivity that hold great promise in the field of cancer immunotherapy.
Previous studies have shown that CIK cells can be generated via incubation of UCB-MNCs with various concentrations of specific cytokines. The classical protocol uses IFN-γ, anti-CD3 antibody, and IL-2, in a complete culture media. Concentrations of these basic cytokines vary across studies; the only common factor is the addition of 1000 U/mL IFN-γ to the culture at day 0. Concentrations of anti-CD3 antibody range from 25 to 50 ng/mL, and those of IL-2 from 300 to 1000 U/mL. Total culturing time also varies from 14 to 28 days Introna et al., 2006Introna et al., 2010Schmidt-Wolf et al.,1991Zhang et al., 2014Zhang et al., 2015.
In our study, we started with the classical protocol of generating CIK cells from the MNCs of three umbilical cord blood samples using RPMI complete medium, then applied four different protocols with regard to concentrations of anti-CD3 antibody and IL-2. All protocols began with the addition of 1000 U/mL IFN-γ to the culture at day 0, followed by supplementation of anti-CD3 antibody and IL-2 on day 1, for a total culture period of 21 days. Concentrations of 25 and 50 ng/mL of Anti-CD3 and 500 and 1000 U/mL of IL-2 were tested in different combinations.
In culture, the addition of IFN-γ prior to IL-2 supplementation has the effect of increasing cytotoxicity; supplementing these cytokines in the reverse order would decrease cytotoxicity Schmidt-Wolf et al., 1991. Anti-CD3 antibody enhances T cell differentiation Lu and Negrin, 1994Ochoa et al., 1987. IL-2 is required for cell proliferation and survival, since in the absence of CD3, cell expansion is greatly limited, and cell death occurs after approximately 7 days in culture Baker et al., 2001. Our results indicate that protocols 3 and 4, with 50 ng/mL of Anti-CD3 and 500 and 1000 U/mL of IL-2 (respectively) yield optimal CIK cell production.
As a heterogeneous population, there are two main subsets in CIK cells: those that are positive for the membrane expression of CD56 (CD3+CD56+) and those that are negative for this marker (CD3+CD56-). However, only cells which express both the T cell marker CD3 and the NK cell marker CD56 have been shown to display anti-tumor capabilities. Immunophenotyping the expanded cells using flow cytometry found CD3+CD56+ subpopulations of 26.08% and 26.21% in experimental groups 3 and 4, respectively, with the highest result being sample 2 in group 4 after a 14-day culture, with a 32.88% CD3+CD56+ subpopulation; these percentages are in accordance with previous studies on CD3+CD56+ CIK cell culture from cord blood samples Introna et al., 2006Zhang et al., 2014Zhang et al., 2015. After 14-21 days in the culture, the number of CD3+CD56+ cells increased by well over a hundred fold, also in accordance with prior reports, which demonstrate that these cells can be expanded up to one thousand fold from both cord blood MNCs Introna et al., 2006 and peripheral blood MNCs Hoyle et al., 1998Kim et al., 2007aKim et al., 2007bOlioso et al., 2009.
Once endowed with NK function, CIK cells also recognize MHC class I-related molecules (MICA and MICB) and members of the UL16 binding protein family (ULBPs) on malignant tumor cells upon interaction with their NKG2D surface receptors, which leads to perforin and granzyme degranulation and tumor cell lysis Mehta et al., 1995Pievani et al., 2011. The induced cells in protocols 3 and 4 both expressed high levels of granzyme B (82.23% and 81.96%). The heterogeneous populations of generated cells included the CD3+CD56+ CIK cells, CD3-CD56+ NK cells, and CD3+CD8+ cytotoxic T lymphocytes; these all contribute to the antitumor activity of the bulk culture.
Another characterization of the antitumor response is the TH1 cytokine release of IFN-γ and TNF-α. IFN-γ, which is a cytokine employed during both innate and adaptive immune responses, produces downstream effects such as increased presentation of class I and II MHC molecules, activation of macrophages, differentiation of naive T cells into TH1 cells, and induction of B cell isotype switching Abbas et al., 2012Aittomäki,2003. TNF-α is a pro-inflammatory cytokine that induces fever and inflammatory responses, apoptosis, and tumor cell necrosis Wajant et al., 2003. At the transcriptional level, the expression of genes that produce these cytokines was higher in the experimental cell populations as compared to fibroblast control samples. Greater production of IFN-γ across MHC barriers can also benefit the preservation of grafts in the presence oftumors without resulting in graftversus- host disease, enhancing the clinical utility of CIK cells in cancer treatment Baker et al., 2001. CIK cells also secrete high levels of IL-2 via overexpression. IL-2 is an essential factor for growth, survival, and differentiation of T lymphocytes Abbas et al., 2012Malek and Bayer, 2004 including NK-T cells. Acting as an autocrine cytokine, IL-2 plays an important role in the generation and proliferation of effector CIK cells. A great deal of prior research has established that CIK cells secrete IFN-γ, TNF-α, and IL-2 Hoyle et al., 1998Kim et al.,2007bWang et al., 2008Zhang et al., 2015.
The cell marker analyses and cytokine production assays from the three different samples in each treatment group revealed that the cytokine concentrations in experimental groups 3 and 4 produced the best results. Both generated large numbers of CIK cells in the presence of anti-CD3 antibody at 50 ng/mL, varying only in IL-2 concentration (500 U/mL and 1000 U/mL, respectively). From a cost-effectiveness standpoint, the group 3 protocol would be more appropriate because of the lower IL-2 concentration used; however, group 4 has the advantage of generating effective CIK cells in a shorter 14-day time period. Recently, researchers have expanded upon classical protocols to attempt to improve CIK cell proliferation and efficacy with additional cytokine inducers such as IL-6, IL-7, IL-15, IL-21, thymoglobulin, or co-culture with dendritic cells Bonanno et al., 2010Heninger et al., 2012Lin et al., 2012Rettinger et al., 2012Schmidt et al., 2004Zhao et al., 2012. More research, including further delineation of CIK cell characteristics, is needed to see if different combinations of these innovative inducers with those outlined in experimental groups 3 and 4 in this study could generate even higher percentages of CD3+CD56+ CIK cells.
Conclusion
In conclusion, the optimal environment for generation of CIK cells from umbilical cord blood in RPMI complete medium is in the presence of IFN-γ at 1000 U/mL, followed 24 hours later by addition of 50 ng/mL of anti-CD3 antibody and either 500 U/mL of IL-2 for a 21-day culture period, or 1000 U/mL of IL-2 for a 14-day culture period. Our results showed high levels of granzyme B-positive CD3+CD56+ subpopulations using both of these protocols. Moreover, these cells demonstrated transcriptional overexpression of the three genes that produce the crucial cytokines IFN- γ, TNF-α, and IL-2. This research is the first to experimentally generate the most effective procedures for generation of CIK cells from umbilical cord bloodderived mononuclear cells, promoting further use of this promising vehicle for immunotherapy research and clinical applications.
References
-
A.K.
Abbas,
A.H.
Lichtman,
S.
Pillai.
Cellular and molecular immunology. Philadelphia: Elsevier/Saunders.
2012
.
-
S.
Aittomäki.
Interferon-gamma-activated gene expression: molecular mechanisms of Stat1-mediated transcription. Tampere University Press.
2003
.
-
A.
Arafar.
Cytokine induced killer cell immunotherapy in cancer treatment: from bench to bedside. Biomedical Research and Therapy.
2014;
1
.
-
J.
Baker,
M.R.
Verneris,
M.
Ito,
J.A.
Shizuru,
R.S.
Negrin.
Expansion of cytolytic CD8+ natural killer T cells with limited capacity for graft-versushost disease induction due to interferon γ production. Blood.
2001;
97
:
2923-2931
.
-
G.
Bonanno,
P.
Iudicone,
A.
Mariotti,
A.
Procoli,
A.
Pandolfi,
D.
Fioravanti,
M.
Corallo,
A.
Perillo,
G.
Scambia,
L.
Pierelli.
Thymoglobulin, interferon-gamma and interleukin-2 efficiently expand cytokine-induced killer (CIK) cells in clinical-grade cultures. Journal of translational medicine.
2010;
8
:
129
.
-
L.
Durrieu,
M.M.
Dieng,
F.
Le Deist,
E.
Haddad.
Cord Blood-Derived and Peripheral Blood-Derived Cytokine-Induced Killer Cells Are Sensitive to Fas-Mediated Apoptosis. Biology of Blood and Marrow Transplantation.
2013;
19
:
1407-1411
.
-
A.-K.
Heninger,
A.
Theil,
C.
Wilhelm,
C.
Petzold,
N.
Huebel,
K.
Kretschmer,
E.
Bonifacio,
P.
Monti.
IL-7 abrogates suppressive activity of human CD4+ CD25+ FOXP3+ regulatory T cells and allows expansion of alloreactive and autoreactive T cells. The Journal of Immunology.
2012;
189
:
5649-5658
.
-
C.
Hoyle,
C.D.
Bangs,
P.
Chang,
O.
Kamel,
B.
Mehta,
R.S.
Negrin.
Expansion of Philadelphia Chromosome-Negative CD3+CD56+ Cytotoxic Cells From Chronic Myeloid Leukemia Patients. In Vitro and In Vivo Efficacy in Severe Combined Immunodeficiency Disease Mice.
1998;
92
.
-
M.
Introna,
M.
Franceschetti,
A.
Ciocca,
G.
Borleri,
E.
Conti,
J.
Golay,
A.
Rambaldi.
Rapid and massive expansion of cord blood-derived cytokineinduced killer cells: an innovative proposal for the treatment of leukemia relapse after cord blood transplantation. Bone marrow transplantation.
2006;
38
:
621-627
.
-
M.
Introna,
A.
Pievani,
G.
Borleri,
C.
Capelli,
A.
Algarotti,
C.
Mico,
A.
Grassi,
E.
Oldani,
J.
Golay,
A.
Rambaldi.
Feasibility and safety of adoptive immunotherapy with CIK cells after cord blood transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow.
2010;
Transplantation16
:
1603-1607
.
-
H.M.
Kim,
J.
Lim,
S.-K.
Park,
J.S.
Kang,
K.
Lee,
C.W.
Lee,
K.H.
Lee,
M.-J.
Yun,
K.-H.
Yang,
G.
Han.
Antitumor activity of cytokine-induced killer cells against human lung cancer. International immunopharmacology.
2007a;
7
:
1802-1807
.
-
H.M.
Kim,
J.
Lim,
Y.D.
Yoon,
J.M.
Ahn,
J.S.
Kang,
K.
Lee,
S.-K.
Park,
Y.J.
Jeong,
J.M.
Kim,
G.
Han.
Anti-tumor activity of ex vivo expanded cytokine-induced killer cells against human hepatocellular carcinoma. International.
2007b;
immunopharmacology7
:
1793-1801
.
-
G.
Lin,
J.
Wang,
X.
Lao,
J.
Wang,
L.
Li,
S.
Li,
J.
Zhang,
Y.
Dong,
A.E.
Chang,
Q.
Li.
Interleukin-6 inhibits regulatory T cells and improves the proliferation and cytotoxic activity of cytokine-induced killer cells. Journal of Immunotherapy.
2012;
35
:
337-343
.
-
Y.C.
Linn,
K.M.
Hui.
Cytokine-induced NK-like T cells: from bench to bedside. BioMed Research International 2010.
2010
.
-
P.-H.
Lu,
R.S.
Negrin.
A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. The Journal of Immunology.
1994;
153
:
1687-1696
.
-
T.R.
Malek,
A.L.
Bayer.
Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol.
2004;
4
:
665-674
.
-
B.A.
Mehta,
I.
Schmidt-Wolf,
I.
Weissman,
R.
Negrin.
Two pathways of exocytosis of cytoplasmic granule contents and target cell killing by cytokine- induced CD3+ CD56+ killer cells. Blood.
1995;
86
:
3493-3499
.
-
Q.
Niu,
W.
Wang,
Y.
Li,
S.
Qin,
Y.
Wang,
G.
Wan,
J.
Guan,
W.
Zhu.
Cord blood-derived cytokine-induced killer cells biotherapy combined with second-line chemotherapy in the treatment of advanced solid malignancies. International immunopharmacology.
2011;
11
:
449-456
.
-
A.
Ochoa,
G.
Gromo,
B.
Alter,
P.
Sondel,
F.
Bach.
Long-term growth of lymphokine-activated killer (LAK) cells: role of anti-CD3, beta-IL 1, interferon-gamma and-beta. The Journal of Immunology.
1987;
138
:
2728-2733
.
-
P.
Olioso,
R.
Giancola,
M.
Di Riti,
A.
Contento,
P.
Accorsi,
A.
Iacone.
Immunotherapy with cytokine induced killer cells in solid and hematopoietic tumours: a pilot clinical trial. Hematological oncology.
2009;
27
:
130-139
.
-
J.R.
Ortaldo,
R.T.
Winkler-Pickett,
H.
Yagita,
H.A.
Young.
Comparative studies of CD3- and CD3+ CD56+ cells: examination of morphology, functions, T cell receptor rearrangement, and pore-forming protein expression. Cellular immunology.
1991;
136
:
486-495
.
-
P.V.
Pham,
B.T.
Vu,
V.Q.
Pham,
P.M.
Le,
H.T.
Le,
N.K.
Phan.
Production of dendritic cells and cytokine-induced killer cells from banked umbilical cord blood samples. Biomedical Research and Therapy.
2015;
2
:
1-7
.
-
A.
Pievani,
G.
Borleri,
D.
Pende,
L.
Moretta,
A.
Rambaldi,
J.
Golay,
M.
Introna.
Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood.
2011;
118
:
3301-3310
.
-
E.
Rettinger,
S.
KuçI,
I.
Naumann,
P.
Becker,
H.
Kreyenberg,
M.
Anzaghe,
A.
Willasch,
U.
Koehl,
G.
Bug,
M.
Ruthardt.
The cytotoxic potential of interleukin-15-stimulated cytokine-induced killer cells against leukemia cells. Cytotherapy.
2012;
14
:
91-103
.
-
D.
Sangiolo,
E.
Martinuzzi,
M.
Todorovic,
K.
Vitaggio,
A.
Vallario,
N.
Jordaney,
F.
Carnevale-Schianca,
A.
Capaldi,
M.
Geuna,
L.
Casorzo.
Alloreactivity and anti-tumor activity segregate within two distinct subsets of cytokine-induced killer (CIK) cells: implications for their infusion across major HLA barriers. International immunology.
2008;
20
:
841-848
.
-
J.
Schmidt,
S.
Eisold,
M.W.
Büchler,
A.
Märten.
Dendritic cells reduce number and function of CD4+ CD25+ cells in cytokine-induced killer cells derived from patients with pancreatic carcinoma. Cancer.
2004;
Immunology
:
Immunotherapy 53, 1018-1026
.
-
R.E.
Schmidt,
C.
Murray,
J.F.
Daley,
S.
Schlossman,
J.
Ritz.
A subset of natural killer cells in peripheral blood displays a mature T cell phenotype. The Journal of experimental medicine.
1986;
164
:
351-356
.
-
I.
Schmidt-Wolf,
P.
Lefterova,
B.
Mehta,
L.
Fernandez,
D.
Huhn,
K.
Blume,
I.
Weissman,
R.
Negrin.
Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Experimental hematology.
1993;
21
:
1673-1679
.
-
I.
Schmidt-Wolf,
R.S.
Negrin,
H.-P.
Kiem,
K.G.
Blume,
I.L.
Weissman.
Use of a SCID mouse/human lymphoma model to evaluate cytokineinduced killer cells with potent antitumor cell activity. The Journal of experimental medicine.
1991;
174
:
139-149
.
-
H.
Wajant,
K.
Pfizenmaier,
P.
Scheurich.
Tumor necrosis factor signaling. Cell Death & Differentiation.
2003;
10
:
45-65
.
-
L.
Wang,
S.
Huang,
Y.
Dang,
M.
Li,
W.
Bai,
Z.
Zhong,
H.
Zhao,
Y.
Li,
Y.
Liu,
M.
Wu.
Cord Blood-Derived Cytokine-Induced Killer Cellular Therapy Plus Radiation Therapy for Esophageal Cancer: A Case Report. Medicine.
2014;
93
:
e34-0
.
-
P.
Wang,
J.-p.
Yu,
S.-y.
Gao,
X.-m.
An,
X.-b.
Ren,
X.-g.
Wang,
W.-l.
Li.
Experimental study on the treatment of intracerebral glioma xenograft with human cytokine-induced killer cells. Cellular immunology.
2008;
253
:
59-65
.
-
Q.
Zhang,
L.
Wang,
C.
Luo,
Z.
Shi,
X.
Cheng,
Z.
Zhang,
Y.
Yang,
Y.
Zhang.
Phenotypic and functional characterization of cytokine-induced killer cells derived from preterm and term infant cord blood. Oncology reports.
2014;
32
:
2244-2252
.
-
Z.
Zhang,
X.
Zhao,
T.
Zhang,
L.
Wang,
L.
Yang,
L.
Huang,
F.
Li,
J.
Liu,
D.
Yue,
F.
Wang.
Phenotypic characterization and anti-tumor effects of cytokine-induced killer cells derived from cord blood. Cytotherapy.
2015;
17
:
86-97
.
-
N.
Zhao,
M.
Zhao,
S.
Rajbhandary,
W.
Lu,
H.
Zhu,
X.
Xiao,
Q.
Deng,
Y.
Li.
Effects of humanized interleukin 21 on anti-leukemic activity of cytokine induced killer cells and the mechanism. Zhonghua xue ye xue za zhi= Zhonghua xueyexue zazhi.
2012;
33
:
823-828
.
Comments
Downloads
Article Details
Volume & Issue : Vol 3 No 01 (2016)
Page No.: 460-468
Published on: 2016-01-29
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 6411 times
- Download PDF downloaded - 1782 times
- View Article downloaded - 5 times
 Biomedpress
Biomedpress