
Expression of epithelial–mesenchymal transition markers in squamous cell carcinoma of the uterine cervix: A cross-sectional study
- Department of Pathology, Sri Devaraj Urs Medical College, Sri Devaraj Urs Academy of Higher Education and research, Kolar, Karnataka, India
- Department of Obstetrics & Gynecology, Sri Devaraj Urs Medical College, Sri Devaraj Urs Academy of Higher Education and research, Kolar, Karnataka, India
- Department of Radiodiagnosis, Sri Devaraj Urs Medical College, Sri Devaraj Urs Academy of Higher Education and research, Kolar, Karnataka, India
Abstract
Background: Cervical cancer is the fourth most common cancer in women worldwide. Epithelial– mesenchymal transition (EMT) is a phenomenon related to carcinogenesis, which is characterized by morphological changes from neoplastic epithelial cells to mesenchymal cells, resulting in increased motility and invasiveness of neoplastic cells. To observe the expression of three EMT markers (cytokeratin 19 [CK1]), vimentin, and Ras homolog gene family member C [RhoC]) in individuals with a normal cervix, patients with high-grade squamous intraepithelial lesion (HSIL), and patients with squamous cell carcinoma (SCC) and determine the association with the histological grade and clinical/radiological stage of cervical cancer.
Methods: Seventy participants were included in the study: 10 individuals with a normal cervix, 30 patients with HSIL, and 30 patients with newly diagnosed SCC. Immunohistochemistry for CK19, vimentin, and RhoC was performed in all groups. The association of the expressions of the three markers between the groups was analyzed. P values of < 0.005 were considered statistically significant.
Results: The mean age of the normal cervix, HSIL, and SCC groups was 46.2 ± 16.12, 49.10 ± 10.13, and 56.27 ± 9.29 years, respectively. CK19 showed a weak basal cell positivity in 80% of the individuals with a normal cervix. Meanwhile, 93.3% and 100% of the patients with HSIL and SCC, respectively, were positive for CK19; the association of the expressions between the groups was insignificant. Vimentin was negative in the normal cervix group and positive in the HSIL (33.3%) and SCC (73.3%) groups; the association of the expressions between the groups was significant. RhoC was positive in the normal cervix, HSIL, and SCC groups (10%, 20%, and 83.33%, respectively), and the association of the expressions between them was significant. There was no significant association found between the expression of vimentin or RhoC and the histopathological grade or FIGO stage.
Conclusion: An increased expression of CK19 in patients with HSIL and SCC of the cervix highlights the role of basal cells in cervical carcinogenesis. The expression of vimentin and RhoC in individuals with a normal cervix and patients with HSIL and SCC indicates EMT.
Introduction
Cervical cancer is the fourth most common cancer in women worldwide after breast cancer, colorectal cancer, and lung cancer. In India, its incidence is 14.7 per 100,000 population, and this type of cancer accounts for approximately 17% of all cancers worldwide1. In Kolar, cervical cancer constitutes 17.55% of all cancers in women2.Squamous cell carcinoma (SCC) accounts for approximately 80% of all cervical cancers and 20% of all adenocarcinomas3.
Cervical cancer occurs in phases. The spectrum of cancer progression includes a normal cervix, low-grade squamous intraepithelial lesion, high-grade squamous intraepithelial lesion (HSIL), locally invasive cancer, and distant metastatic cancer4.
During the progression of epithelial cancer, epithelial cells lose their own cell characteristics and acquire mesenchymal cell characteristics. This phenomenon is called the epithelial–mesenchymal transition (EMT), which is the most important mechanism for local and distant metastases and determines the prognosis of cancer5, 6.
EMT is widely studied in patients with head and neck cancer and breast cancer. However, only few studies on EMT in patients with cervical cancer are published in the English literature. Hence, this study evaluated the expressions of cytokeratin 19 (CK19) as an epithelial marker, vimentin as a mesenchymal modulator, and Ras homolog gene family member C (RhoC) as an EMT activator and cytoskeletal modulator.
Methods
The present study had a cross-sectional design and was approved by our institutional ethical committee. A total of 70 cervical biopsy specimens—normal cervix (10 cases), HSIL (30 cases), and SCC (30 cases)—were collected from the department of pathology of a tertiary health care center in South India. Newly diagnosed HSIL and SCC were included in the study. Meanwhile, cases treated with chemotherapy or radiotherapy, malignancy other than SCC of the cervix, recurrent cases of cervical cancer, and metastatic deposits in the cervix were excluded from the study.
The sociodemographic and clinical characteristics of the patients, including age, marital status, parity, presenting symptoms, and signs based on abdominal, vaginal, and speculum examinations were collected from case files. The histological grade and stage of carcinoma were also noted. SCC was histologically graded as well differentiated, moderately differentiated, and poorly differentiated. FIGO staging was considered in the study7.
The formalin-fixed, paraffin-embedded tissues of all cases in the three groups were sectioned to a thickness of 4 µm. The expressions of CK19, vimentin, and RhoC were determined via immunohistochemistry (IHC). The primary antibodies used in IHC were mouse monoclonal antibody for CK19 (Biogenex), mouse monoclonal antibody for vimentin (Biogenex), and rabbit polygonal antibody for RhoC (Immunotag). Positive and negative controls were utilized for each batch of staining.
Cytoplasmic and/or membrane staining of the cells for CK19 was considered positive. The percentage of cells staining positive for CK19 was scored (proportion score) as follows: 0 when <10% of the cells showed positivity; 1, 10% to 25%; 2, 26% to 50%; 3, 51% to 75%; and 4, >76%. The staining intensity (intensity score) was graded as 0 for negative staining, 1 for weak staining, 2 for moderate staining, and 3 for strong staining. The final IHC score was calculated by multiplying the proportion score with the intensity score. The final immunoreactivity score was graded as 0 for negative staining (final IHC score = 0), 1+ for weakly positive staining (IHC score = 1 to 4), 2+ for moderately positive staining (IHC score = 5 to 8), and 3+ for strongly positive staining (IHC score = 9 to 12)8.
Vimentin was considered positive in the presence of cytoplasmic staining. The proportion score was considered 0 when 0% of the cells showed positivity; 1, 1% to 10%; 2, 11% to 40%; 3, 41% to 75%; and 4, 76% to 100%. The intensity score was calculated as 0 when the cells were colorless; 1 when the cells stained light yellow; 2 when the cells stained brown yellow; and 3 when the cells stained dark brown. The final immunoreactivity score was calculated by multiplying the proportion score with the intensity score. The immunoreactivity was considered negative for a final score of 0 or 1, weakly positive for 2 or 3, positive for 4 – 7, and strongly positive for 8 — 129.
RhoC was also considered positive in the presence of cytoplasmic staining. The proportion score was calculated as 0 when 0% of the cells showed positivity; 1, 0% to 25%; 2, 26% to 50%; 3, 51% to 75%; and 4, 76% to 100%. The intensity score was considered 0 when the cells were colorless; 1 when the cells showed a faint intensity; 2 when the cells demonstrated a moderate intensity; and 3 when the cells showed a strong intensity. The final immunoreactivity score was calculated by multiplying the proportion score with the intensity score. The cells were considered positive for RhoC with a final immunoreactivity score of ≥6. The final immunoreactivity score was graded as 0 for negative staining (IHC score of 0 or 1), 1+ for equivocal staining (IHC score of 2–5), 2+ for moderately positive staining (IHC score of 6–8), and 3+ for strongly positive staining (IHC score of 9–12). An equivocal reaction was considered negative for RhoC10.
Data were entered into Microsoft Excel datasheets and analyzed using SPSS 22.0. Continuous variables were presented as frequencies, percentages, means, and standard deviations. Analysis of variance was performed to test the significance and identify the mean difference between more than two groups of quantitative data. The chi-square test or Fisher’s exact test was used to test the significance of qualitative data. P values of <0.005 were considered statistically significant.
Results
The mean age in the normal cervix, HSIL, and SCC groups was 46.2 ± 16.12, 49.10 ± 10.13, and 56.27 ± 9.29 years, respectively. The age distribution significantly differed between the three groups (p = 0.001). Among the SCC cases, 46.66% (n = 14), 26.67% (n = 8), and 26.67% (n = 8) were moderately differentiated, well differentiated, and poorly differentiated, respectively. Approximately 6.6% (n = 2), 63.3% (n = 19), 26.6% (n = 8), and 3.3% (n = 1) were categorized under stages I, II, III, and IV, respectively.

CK19 basal cell positivity in normal cervix (CK19, X400).
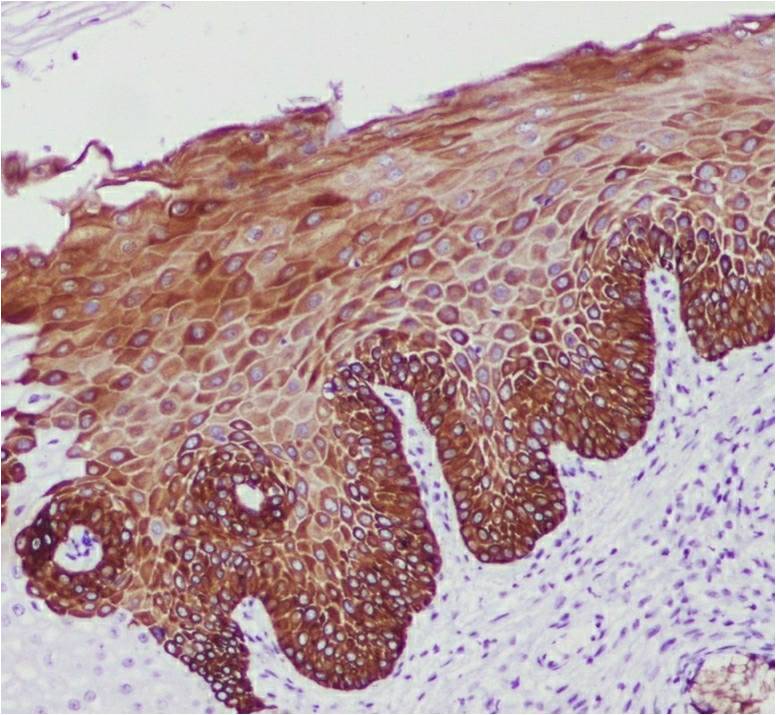
CK19 strong positive expression in HSIL (CK19,X400).

CK19 strong positive expression in squamous cell carcinoma cervix (CK19, X400).

Negative expression of Vimentin in epithelial component of normal cervix. (Vimentin, X100).
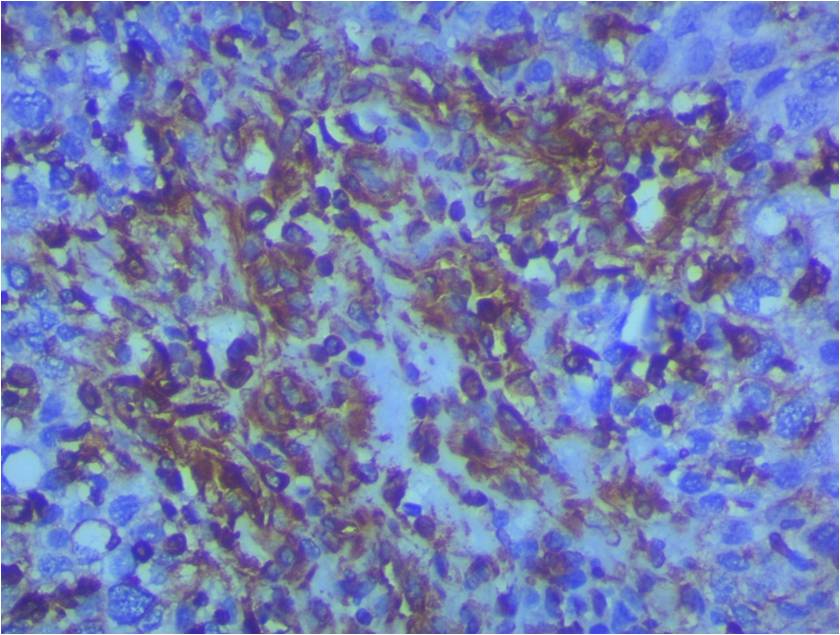
Vimentin showing positive expression in SCC. (Vimentin, X400).

Negative expression of RhoC in normal cervix. (RhoC, X100)

RhoC showing positive expression in squamous cell carcinoma cervix.(RhoC, X400)
Expressions of CK19, Vimentin and RhoC in normal, HSIL and SCC cervix
|
Markers |
Normal n (%) |
HSIL n (%) |
SCC n (%) |
|
p Value |
|---|---|---|---|---|---|
|
CK19 | |||||
|
Positive |
08 (80%) Weak Basal |
28 (93.3%) |
30 (100%) |
- |
0.492 |
|
Negative |
02 (20%) |
02 (6.7%) |
00 (00%) | ||
|
Vimentin | |||||
|
Positive |
00 (00%) |
10 (33.3%) |
22 (73.3%) |
19.496 |
<0.001 |
|
Negative |
10 (100%) |
20(66.7%) |
08(26.7) | ||
|
RhoC | |||||
|
Positive |
01(10%) |
06 (20%) |
25(83.33%) |
30.241 |
<0.001 |
|
Negative |
09(90%) |
24(80%) |
05(16.67%) |
Expressions of Vimentin and RhoC in different histological grades and FIGO stages of SCC
|
Vimentin |
P value |
RhoC |
P value | |||
|---|---|---|---|---|---|---|
|
Positive |
Negative |
Positive |
Negative | |||
|
Histological grades | ||||||
|
WDSCC n (%) |
05 (62.5%) |
03 (37.5%) |
0.515 |
07 (87.5%) |
01 (12.5%) |
0.827 |
|
MDSCC n (%) |
10 (71.43%) |
04 (28.57%) |
11 (78.57%) |
03 (21.43%) | ||
|
PDSCC n (%) |
07 (87.5%) |
01 (12.5%) |
07 (87.5%) |
01 (12.5%) | ||
|
FIGO Stages | ||||||
|
I |
00 |
02 (100%) |
0.589 |
00 |
02 (100%) |
0.593 |
|
II n (%) |
15 (78.9%) |
04 (21.0%) |
16 (84.2%) |
03 (15.7%) | ||
|
III n (%) |
05 (62.5%) |
03 (37.5%) |
07 (87.5%) |
01 (12.5%) | ||
|
IV |
01 (100%) |
00 |
01 (100%) |
00 | ||
Of the cases with a normal cervix, CK19 showed a weak basal positivity in 80% (n = 8) and negativity in 20% (n = 2) (Figure 1). Among the HSIL cases, 93.3% (n = 28) and 6.7% (n = 2) were positive and negative for CK19, respectively (Figure 2). Of the 93.3% (n = 28) of CK19-positive HSIL cases, 3.3% (n = 10) showed a strong positive expression; 10% (n = 10), moderately positive expression; and 80% (n = 24), weakly positive expression. The expression of CK19 was increased in the basal and intermediate layers of the cervix in the HSIL group compared with that in the normal cervix group. All SCC cases were positive for CK19, of which 56.7% (n = 17) were strongly positive; 33.3% (n = 10) moderately positive; and 10% (n = 3) weakly positive (Figure 3). The CK19-positive cells in the SCC group were observed up to the superficial layer compared with those in the normal cervix and HSIL groups. There was no significant association found in the CK19 expression between the HSIL and SCC groups (p = 0.492) (
All cases (n = 10) with a normal cervix were negative for vimentin (
Approximately 90% (n = 9) of the cases with a normal cervix were negative for RhoC, while one case was strongly positive for RhoC (Figure 6). Meanwhile, 20% (n = 6) of the HSIL cases were positive for RhoC, of which 13.3% (n = 4) showed strongly positive staining, and 6.7% (n = 2) demonstrated moderately positive staining. Approximately 73.3% (n = 22) of the HSIL cases were negative for RhoC, while 6.7% (n = 2) showed an equivocal immunopositivity. An equivocal immunopositivity was considered negative for RhoC. Of the SCC cases, 83.33% (n = 25) were positive for RhoC, of which 50% (n = 15) were strongly positive, and 33.33% (n = 10) were moderately positive (Figure 7). The association of the expression of RhoC between the normal cervix, HSIL, and SCC groups was significant (p < 0.001) (
Of the well-differentiated SCC cases, 62.5% (n = 5) and 37.5% (n = 3) were positive and negative for vimentin, respectively. Approximately 71.43% (n = 10) and 28.57% (n = 4) of the moderately differentiated SCC cases were immunopositive and immunonegative for vimentin, respectively. Among the poorly differentiated SCC cases, 87.5% (n = 7) showed immunopositivity, and 12.5% (n = 1) showed immunonegativity. A positive expression of vimentin increased the grade of SCC; however, the association between the different grades of SCC was not significant (p = 0.515). Approximately78.9% (n = 15) of the cases under stage II, 62.5% (n = 5) under stage II, and 100% (n = 1) under stage IV showed positive expressions of vimentin. In contrast, 100% (n = 2) cases under stage I, 21.0% (n = 4) under stage II, and 37.5% (n = 3) under stage III showed negative expressions of vimentin. The association of the expressions of vimentin between the FIGO stages was not significant (p = 0.589) (
Among the well-differentiated SCC cases, 87.5% (n = 7) were positive for RhoC, while 12.5% (n = 1) were negative for RhoC. Of the moderately differentiated SCC cases, 78.57% (n = 11) were immunopositive for RhoC, and 21.43% (n = 3) were immunonegative for RhoC. Approximately 87.5% (n = 0) and 12.5% (n = 1) of the poorly differentiated cases showed immunopositivity and immunonegativity for RhoC, respectively. The association of the expressions of RhoC between the different grades of SCC was not significant (p = 0.827). Meanwhile,84.2% (n = 16) of the cases under stage II, 87.5% (n = 7) under stage III, and 100% (n = 1) under stage IV showed positive expressions of RhoC. Further, 100% (n = 2) of the cases under stage I, 15.7% (n = 3) under stage II, and 12.5% (n = 1) under stage III showed negative expressions of RhoC. The association between the FIGO stages and RhoC expression was insignificant (p = 0.593) (
CK19 showed 100% positivity in all SCC cases. Thus, a comparison of the expression of CK19 between the histological grades and FIGO stages was not possible.
Discussion
Cervical cancer is the fourth most common cancer among women worldwide. Among Indian women, cervical cancer accounts for 16.5% of all cancers and is the second most common cause of death due to cancer1. Advanced age, low socioeconomic status, high parity, improper hygiene, multiple partners, and HPV infections are common risk factors. Cervical cancer occur in a step-wise pattern from normal tissue to HSIL and finally SCC4.
Epithelial cells have apical–basal polarity and are connected by a specialized adhesion complex, while mesenchymal cells are spindle shaped and more motile with a front-to-back cell polarity and lack adhesion complexes. Epithelial cells can be converted into mesenchymal cells via EMT. During EMT, suppression of epithelial adhesion junctions, gain of mesenchymal markers, cytoskeleton reorganization, anoikis resistance, and increased cellular migration and invasiveness are observed10, 11.
CK19 is basal cell marker. In the present study, 80% of the normal cervical biopsy specimens (n = 10) showed a weak basal cell positivity for CK19, while 20% showed a negative expression. Approximately 93.3% (n = 28) of the HSIL cases showed immunopositivity for CK19, of which 80% (n = 24) demonstrated weak positivity. During transformation from normal tissue to HSIL, more CK19-positive cells appear in the intermediate cell layer. This finding is comparable to that reported by Lee . where 60% (n = 15) of HSIL cases showed diffuse staining with CK1911. Herein, 100% (n = 30) of the SCC cases were positive for CK19, also consistent with the findings by Lee . (n = 30)12. Approximately 37% (n = 11) of SCC cases showed diffuse positivity for CK19 in the study by Lee .12. In the present study, 56.7% (n = 17) showed a strong positive expression of CK19. During transformation from HSIL to SCC, more CK19-positive cells migrated to the intermediate and superficial layers of the cervix. The difference in the expression among studies may be because of the different methods used for the interpretation of CK19 immunoexpression and heterogeneity in tumor cells12.
Vimentin is a structural protein that belongs to type III intermediate filament proteins expressed in mesenchymal cells and has a role in cytoskeletal formation and thus provides mechanical resistance to cells. It is also involved in organelle positioning, cell migration, cell adhesion, and cell signaling pathways13. In the present study, 0% of the normal cervical biopsy samples, 33.3% (n = 10) of the HSIL samples, and 73.3% (n = 22) of the SCC samples showed immunopositivity for vimentin. There was increase in the expression of vimentin from normal tissue to HSIL and SCC. This finding is similar to that by Myong . and Jiang .14, 15.
RhoC belongs to the Rho GTPase family. It is an effector of transcription modulator Notch1 through the phosphatidyl inositol-3 kinase pathway in cervical cancer16. This marker regulates actin organization in tumors, resulting in enhanced migration, invasion, and metastasis. RhoC mediates EMT, which is stimulated by growth factors such as TGF-β1. It regulates the mitogen activated protein kinase and phosphatidylinositide 3 kinase/AKT serine threonine kinase (PI3K/AKT) pathways, which are involved in cancer progression and maintenance. RhoC also plays a role in angiogenesis by modulating the expressions of growth factors required in angiogenesis, including vascular endothelial growth factor, fibroblast growth factor, interleukin 6, and interleukin 8. The involvement of RhoC in the stemness of cancer cells is also recently reported17. Targeted therapy against RhoC has been currently developed by targeting an HLA restricted epitope of RhoC. Apart from targeted therapy and immunotherapy against RhoC, statins reverse RhoC-induced tumor phenotypes. These drugs are inhibitors of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG- CoA reductase), which contributes to the HMG-CoA reductase pathway. This pathway produces intermediate products, such as geranylgeranyl pyrophosphate and farnesyl pyrophosphate, which activate Rho GTPases18, 19, 20. Herein, 83.3% (n = 25) of the SCC cases showed a positive expression of RhoC as compared with 10% (n = 1) and 20% (n = 6) of the cases with a normal cervix and HSIL cases, respectively. The SCC cases had a higher expression of RhoC than the other cases. These findings are similar to those reported by Nai et al., Srivastava ., and Tanaka .16, 21, 22. RhoC expression was observed to increase from normal tissue to SCC in the present study, consistent with the findings by Nai . and Srivastava .16, 21.
In the current study, 87.5% (n = 7) of the poorly differentiated SCC cases showed positivity for vimentin. The expression of vimentin increased in the poorly differentiated SCC cases compared with that in the well- and moderately differentiated SCC cases, comparable with the findings by Yu.23. Meanwhile, a reduced expression of vimentin (36%) was observed by Lin .24. The expression of vimentin increased with the grade of cancer from well-differentiated SCC to moderately and poorly differentiated SCCs. However, the difference in the expression of vimentin across the tumor grades was insignificant. Approximately 78.9% (n = 15) of the cases under stage II, 62.5% (n = 5) under stage II, and 100% (n = 1) under stage IV showed positive expressions of vimentin. There was no significant association found between vimentin expression and FIGO stage. This finding is similar to that by Yu ., Li ., and Lin .; in their studies, vimentin expression was observed in the early stages23, 24, 25.
Herein, the maximum expression of RhoC was seen in the well-differentiated and poorly differentiated SCCs. However, the expression of RhoC among the grades of cervical cancer was insignificant, similar to the finding by Nai .21. Approximately 84.2% (n = 16) of the cases under stage II, 87.5% (n = 7) under stage III, and 100% (n = 1) under stage IV showed a positive expression of RhoC. There was no significant association found between RhoC expression and FIGO stage, consistent with the findings by Nai . and Tanaka 21, 22.
The limitations of the present study are the unicentric study design and small sample size of the SCC cases. However, CK19 expression highlights the role of basal cells in cervical carcinogenesis. An increase in the expression of vimentin and RhoC from normal tissue to HSIL and SCC indicates EMT. Vimentin and RhoC show maximum expression in poorly differentiated SCC. RhoC expression is higher with a higher stage of SCC. Hence, vimentin and RhoC can be considered as predictors of the progression/prognosis of cervical neoplasia; this finding can be utilized for adjuvant targeted chemotherapy. Further multicentric studies with a larger sample size are required to extrapolate the findings to the general population, especially the utilization of RhoC in adjuvant targeted therapy for cervical cancer.
Conclusions
An increased expression of CK19 in patients with HSIL and SCC of the cervix highlights the role of basal cells in cervical carcinogenesis. A gradual increase in the expressions of vimentin and RhoC from a normal cervix to HSIL and SCC of the cervix indicates EMT, which eventually increases the motility and invasiveness of tumor cells in cervical cancer.
Abbreviations
CK: Cytokeratin, EMT: Epithelial mesenchymal transition, FIGO: International federation of gynaecology and obstetrics, HMG-CoA: 3-hydroxy 3-methyl glutaryl coenzyme A, HPV: Human Papilloma Virus, HSIL: High grade squamous intraepithelial lesion, IHC: Immunohistochemistry, RhoC: Ras homolog gene family member C, SCC: Squamous cell carcinoma, TGF: Transforming growth factor
Acknowledgments
None.
Author’s contributions
AA: Data collection, manuscript writing, KR: Concept, manuscript editing, manuscript reviewing, SSR: Manuscript writing, AKS: Manuscript writing. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
None.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board approved the study, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

