Abstract
Background: Acute myeloid leukemia (AML) with t(8;21)(q22;q22) is a frequently encountered subtype of AML with recurrent genetic abnormalities, found in approximately 1?5% of AML cases. Here, we present cases of AML with t(8;21) in elderly patients with aberrant B-marker expression identified at our institution, including their clinical outcomes when treated with hypomethylating agents and BCL-2 inhibitors.
Case presentation: A 60-year-old patient diagnosed with AML carried the t(8;21) chromosomal translocation. Immunophenotyping and immunohistochemistry revealed aberrant expression of B-markers, including CD19, CD79a, and PAX5. Cytogenetic analysis also identified a loss of the X chromosome, a common cytogenetic aberration in AML associated with t(8;21). Due to the patient's age and inability to tolerate intensive chemotherapy, treatment was initiated using a hypomethylating agent and a BCL-2 inhibitor. Although the initial bone marrow evaluation showed an excess of blast cells, subsequent assessments demonstrated a favorable response to the treatment, with the absence of blast cells and improvements in peripheral blood parameters.
Conclusion: The presence of B-marker expression in AML with t(8;21) is a relatively common occurrence. The integration of cytogenetic and molecular investigations plays a vital role in accurately diagnosing and classifying AML. A remarkable feature of AML with t(8;21) is its high remission rate, and this holds true even in cases where standard intensive chemotherapy is not utilized. Moreover, the detection of aberrant B-marker expression, particularly CD19, signifies a favorable prognosis.
Introduction
Acute myeloid leukemia (AML) with t(8;21)(q22;q22.1) is one of the most frequently identified subtypes of AML and is characterized by recurrent genetic abnormalities. This particular genetic alteration accounts for approximately 1–5% of all AML cases. Notably, AML with t(8;21)(q22;q22.1) primarily affects children and younger individuals, while its occurrence is relatively uncommon among individuals over the age of 601.
The translocation of chromosomes 8:21 involves the fusion of two specific genes, namely, the ETO gene (also known as RUNX1T1) located on chromosome 8 and the AML1 gene (also known as RUNX1) located on chromosome 21. This reciprocal translocation event results in the rearrangement and subsequent fusion of these two genes, forming the RUNX1–RUNX1T1 fusion protein. This fusion protein functions as a transcriptional repressor, disrupting the normal processes of myeloid cell differentiation and maturation. An abnormal accumulation of immature myeloid cells consequently ensues, contributing to the development of AML2.
AML with t(8;21)(q22;q22) has demonstrated more favorable prognoses, particularly when treated with high-dose cytarabine-based therapy, supported by studies such as that by Wilde et al.3. However, due to its potential side effects, this intensive chemotherapy regimen is not recommended for the elderly population4.
Case Presentation
A 60-year-old woman presented to the clinic with symptoms of lethargy, pallor, and spontaneous bruising that had been present for one week. She had previously been in good health and had no known medical conditions. No fever was apparent, and she had sustained no recent trauma nor recent blood loss.
During the examination, she was alert and conscious but appeared pale. There was no jaundice observed. Her vital signs were within the normal range, with normotensive blood pressure and a heart rate of 90 beats per minute. She did not have a fever, and there were no signs of lymphadenopathy, hepatomegaly, or splenomegaly. Several bruises, measuring approximately 1 to 2 cm each, were noted on both forearms. Other systemic examinations did not reveal any notable findings.
Laboratory investigation
The initial full blood count revealed the following abnormalities: leucocytosis with a white cell count of 44.2 x 109/L, anemia with a hemoglobin level of 3.3 g/dL, and thrombocytopenia with a platelet count of 7 x 109/L. Meanwhile, the absolute neutrophil count was within the reference range at 5.0 x 109/L.
The coagulation profile showed a normal prothrombin time of 15.1 seconds (reference range: 12.6 – 15.7 seconds) and a normal activated partial thromboplastin time of 32.8 seconds (reference range: 30 – 45.8 seconds). The international normalized ratio (INR) was 1.12, indicating normal clotting function. There was no evidence of disseminated intravascular coagulopathy, as the fibrinogen levels were normal. However, the D-dimer level was mildly elevated at 0.91 µg/mL (reference range: < 0.45 µg/mL).
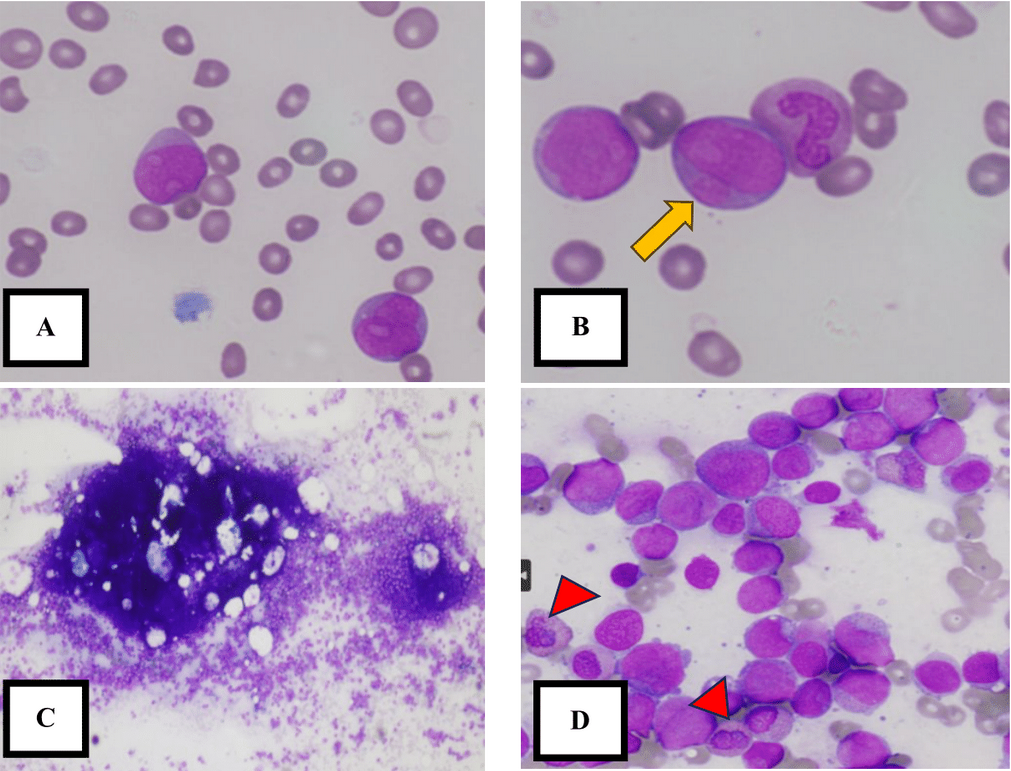
Suspecting a hematological malignancy, an urgent full blood picture was requested, revealing the presence of 78% blast cells (Figure 1). The following day, bone marrow aspiration was performed at the posterior superior iliac spine. The fragments and cell trails were hypercellular, consisting of a homogeneous population of blast cells. These blast cells accounted for 86.4% of the sample and were of moderate to large size, displaying open chromatin, prominent nucleoli, a round to irregular nuclear outline, and scant cytoplasm. Some blast cells exhibited cytoplasmic granules, while others showed the presence of Auer rods. Other granulocytic precursors were suppressed, and there was marked suppression of erythropoiesis, lymphopoiesis, and megakaryopoiesis (Figure 1).
Trephine biopsy also revealed hypercellular marrow with diffuse infiltration of blast cells. The blasts were positive for CD34 and CD117, with dim expression of CD19 and PAX 5. Marked suppression of other hematopoietic cell lineages was observed (Figure 2).
Immunophenotyping by flow cytometry (Figure 3) revealed that the blast population expressed dim to positive CD45 (78.9%-88.5%) and showed low to moderate side scatter (SSC). The blast cells exhibited heterogeneous expression of CD34 and positive MPO, CD13, HLA-DR, CD64, CD38, CD58, CD123, and CD9 (20.1%). Aberrant expression of CD19 (54.8%) and CD79a (13%) was also observed. However, they tested negative for T-cell markers and monocytic markers.
Cytogenetic analysis of all examined cells (12 metaphases) revealed the presence of abnormal metaphases, indicating an abnormal clone. The abnormal clone consisted of 45 chromosomes, with the loss of one X chromosome and a reciprocal translocation between chromosome 8 at segment 8q22 and chromosome 21 at segment 21q22. Molecular analysis using qualitative HemaVision-28 N translocation confirmed the presence of t(8;21) (q22;q22) involving the RUNX1–RUNX1T1 fusion gene (Figure 4).
Based on the findings from the marrow aspirate, trephine biopsy, and molecular analysis, the patient’s final diagnosis was AML with aberrant B-marker expression.
Outcome
During her first admission, the patient received a total of 12 units of platelets and 4 units of red blood cells. After the diagnosis was established and she received counseling, she agreed to undergo treatment with a hypomethylating agent (azacitidine) and a BCL-2 inhibitor (venetoclax). Following the first cycle of treatment with azacitidine, the patient's full blood count showed pancytopenia, and the bone marrow continued to exhibit an excess of blast cells. However, after the second cycle of treatment, her hemoglobin and platelet count improved and returned to the normal range. Additionally, the bone marrow showed signs of remission. At present, the patient has completed four cycles of treatment and is scheduled to undergo a haploidentical allogeneic transplant in the future.
Discussion
Our case highlights AML with aberrant expression of the B-cell markers CD19, PAX5, and CD79a. In AML with t(8;21), leukemic blasts may exhibit lymphoid markers such as CD19 and PAX5, as well as cytoplasmic expression of CD79a1. In a study conducted in India involving 29 patients diagnosed with AML and t(8;21), aberrant expression of CD19 was observed in 44.8% of patients5.
The initial challenge we faced upon receiving the bone marrow sample and conducting immunophenotyping was distinguishing between AML and mixed phenotypic acute leukemia (MPAL). This differentiation is crucial, as AML with t(8;21) and MPAL have distinct prognoses. AML with t(8;21) is characterized by relatively favorable outcomes and a high rate of complete remission. In contrast, MPAL is associated with poor prognoses1.
According to the World Health Organization (WHO) classification 2016, MPAL of the B/myeloid lineage requires the presence of myeloperoxidase (MPO) or monocytic differentiation, along with strong CD19 expression or weak CD19 expression accompanied by strong expression of at least two of CD79a, CD22, and CD10. In our case, we observed strong CD19 expression, dim CD79a expression, and negative expression of other B-markers, such as CD10, CD20, and CD22, which makes diagnosis of MPAL less likely.
We also observed PAX5 expression in leukemic blasts. Walter et al. conducted a study in which they found coexpression of CD19 and PAX5 in AML blast cells with t(8;21). That study revealed that PAX5 directly binds to the CD19 promoter, acting as a major regulator of CD19 expression. These findings suggest that the accessibility of the chromatin structure in the CD19 gene is essential for PAX5-mediated CD19 activation. However, the specific mechanisms through which PAX5 itself is upregulated in primary t(8;21) cells remain unknown6.
Based on the molecular report and cytogenetic analysis, which revealed the presence of t(8;21), we can confidently diagnose the patient with AML exhibiting aberrant B-marker expression. This finding also highlights the significance of incorporating molecular and cytogenetic analysis into the diagnostic process for AML. It is important to note that the diagnosis of AML with recurrent genetic abnormalities does not necessitate the presence of blasts exceeding 20%1.
In addition to the t(8;21) cytogenetic aberration, the patient also exhibited loss of the X chromosome. In AML with t(8;21), the loss of X chromosome was observed in 30–40% of females, while loss of the Y chromosome occurs in 50% of males. The loss of X chromosome does not influence the positive prognosis, however, the absence of the Y chromosome is associated with better outcome7.
A systematic review investigating aberrant phenotypic expression in AML patients concluded that CD19 expression has a positive impact on prognosis8. Several studies have found that CD19 expression in AML patients is associated with a favorable prognosis, as these patients achieved complete remission8.
Our patient was treated with a combination of a hypomethylating agent and a BCL-2 inhibitor. The treatment was selected based on the patient's age and the determination that she was unfit for intensive chemotherapy. Although the initial response after treatment revealed an excess of blast cells in the bone marrow, subsequent assessments showed no excess of blast cells and demonstrated improvements in the peripheral white blood cell count, hemoglobin levels, and platelet count. These findings indicate a positive response to the treatment administered.
Conclusion
In conclusion, it is not uncommon for AML with t(8;21) to involve B-marker expression. The incorporation of additional cytogenetic and molecular studies is crucial for the diagnosis and classification of AML. A notable characteristic of AML with t(8;21) is its high remission rate, even in cases where standard intensive chemotherapy is not administered. Furthermore, the presence of aberrant B-marker expression, including CD19, is indicative of a favorable prognosis.
Abbreviations
AML: Acute myeloid leukemia, MPAL: Mixed phenotypic acute leukemia, WHO: World Health Organization
Acknowledgments
We are grateful for our laboratory staff at the Department of Hematology, Hospital Universiti Sains Malaysia for their great assistance.
Author’s contributions
The initial concept is by Nur Ilyia Syazwani, Noor Haslina Mohd Noor. The first draft is wrote by Nur Ilyia Syazwani, Abdul Hanan Abdullah. Razan Hayati Zulkeflee, contributed to the cytogenetic and molecular analysis result. Mohd Nazri Hassan, Marne Abdullah, Nurul Asyikin Nizam Akbar contributed to the immunophenotyping, bone marrow aspirate and trephine biopsy result. Nur Ilyia Syazwani, Noor Haslina Mohd Noor revising it critically for important intellectual content. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki. The institutional review board approved the study, and the participant provided written informed consent.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Steven
H.,
WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. Revised 4th Edition. International Agency for Reasearch on cancer (IARC); 2017. 130–132 p. . 2017
.
-
Reikvam
H.,
Hatfield
K.J.,
Kittang
A.O.,
Hovland
R.,
Bruserud
O.,
Acute myeloid leukemia with the t(8;21) translocation: clinical consequences and biological implications. Journal of Biomedicine & Biotechnology.
2011;
2011
:
104631
.
View Article PubMed Google Scholar -
Wilde
L.,
Cooper
J.,
Wang
Z.X.,
Liu
J.,
Clinical, Cytogenetic, and Molecular Findings in Two Cases of Variant t(8;21) Acute Myeloid Leukemia (AML). Frontiers in Oncology.
2019;
9
:
1016
.
View Article PubMed Google Scholar -
Di Francia
R.,
Crisci
S.,
De Monaco
A.,
Cafiero
C.,
Re
A.,
Iaccarino
G.,
Response and Toxicity to Cytarabine Therapy in Leukemia and Lymphoma: From Dose Puzzle to Pharmacogenomic Biomarkers. Cancers (Basel).
2021;
13
(5)
:
966
.
View Article PubMed Google Scholar -
Baul
S.N.,
Baveja
A.,
Mandal
P.K.,
De
R.,
Dutta
S.,
Dolai
T.K.,
A glimpse into translocation (8;21) in acute myeloid leukemia: profile and therapeutic outcomes from a tertiary care hematology center from East India. Journal of Hematology and Allied Sciences..
2022;
2
:
85-90
.
View Article Google Scholar -
Walter
K.,
Cockerill
P.N.,
Barlow
R.,
Clarke
D.,
Hoogenkamp
M.,
Follows
G.A.,
Aberrant expression of CD19 in AML with t(8;21) involves a poised chromatin structure and PAX5. Oncogene.
2010;
29
(20)
:
2927-37
.
View Article PubMed Google Scholar -
Mohamed
M.,
Dun
K.,
Acute myeloid leukaemia with t(8;21)(q22;q22.3) and loss of the X chromosome. BMJ Case Reports.
2015;
2015
.
View Article PubMed Google Scholar -
Pinheiro
L.H.,
Trindade
L.D.,
Costa
F.O.,
Silva
N.L.,
Sandes
A.F.,
Nunes
M.A.,
Aberrant Phenotypes in Acute Myeloid Leukemia and Its Relationship with Prognosis and Survival: A Systematic Review and Meta-Analysis. International Journal of Hematology-Oncology and Stem Cell Research.
2020;
14
(4)
:
274-88
.
PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 8 (2023)
Page No.: 5804-5809
Published on: 2023-08-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4583 times
- PDF downloaded - 1435 times
- XML downloaded - 113 times
 Biomedpress
Biomedpress






