Abstract
Introduction: Nasopharyngeal carcinoma (NPC) is a common malignancy in Viet Nam, and its pathogenesis is closely associated with Epstein?Barr virus (EBV) infection. However, the relationship between EBV infection and clinicopathological characteristics related to NPC prognosis in Vietnamese patients remains poorly understood. This study aimed to investigate the association between EBV infection and various clinical parameters in Vietnamese patients with NPC.
Methods: We collected clinical data from 31 patients with histologically confirmed NPC and evaluated their samples for EBV-encoded RNA (EBER) expression using the chromosomal in situ hybridization (CISH) technique. We examined the relationship between EBER expression and several clinical parameters, including age, sex, lymph node metastasis, tissue invasion and metastasis, clinical stage, and histological NPC type.
Results: The patients' ages ranged from 19 to 74 years, with 80.64% aged >40 years and 87.1% male. In addition, 80.65% had lymph node metastasis, and 38.71% had tissue invasion and distant metastasis. Most patients (67.74%) were diagnosed at a late stage (III or IV), with the most common histological type being type III (48.39%), followed by type I (29.03%) and II (22.58%). EBER expression was observed in 48.39% of the patients and was significantly associated with younger age (<40, p = 0.0362) and undifferentiated carcinoma (type III, p = 0.0007). However, EBER expression was not significantly associated with sex, lymph node metastasis, tissue invasion and metastasis, or clinical stage.
Conclusions: Our study suggests that EBV infection may contribute to NPC pathogenesis. It also shows significant associations between EBV infection and younger age and undifferentiated carcinoma type. The CISH technique could help screen asymptomatic high-risk individuals, managing and predicting the NPC prognosis. The small sample size and single-center design limit the generalizability of our findings.
Introduction
Nasopharyngeal carcinoma (NPC) is a rare cancer worldwide but is more common in the Southern provinces of China, Southeast Asia, and Vietnam1. The Global Cancer Observatory (GLOBCAN) 2020 estimates of cancer incidence and mortality indicate that NPC is the ninth most common cancer in Vietnam, with an average incidence of 16.63 per 100,000 population2. The World Health Organization (WHO) classification divides NPC into two main histological groups: keratinizing squamous cell carcinoma (type I) and non-keratinizing squamous cell carcinoma (types II and III). The non-keratinizing group is further divided into two subgroups: differentiated non-keratinizing carcinoma (type II) and undifferentiated carcinoma (type III)3.
The etiologies and risk factors for NPC vary geographically. The primary risk factor for NPC in high endemic areas is Epstein–Barr virus (EBV) infection, followed by genetic factors and lifestyle habits4. In particular, EBV plays a significant role in NPC pathogenesis5, 6.
Various techniques are available to identify EBV infection, including immunohistochemical (IHC) staining, chromosomal in situ hybridization (CISH), and polymerase chain reaction (PCR). CISH is optimal for detecting EBV infection in formalin-fixed paraffin-embedded tissue due to its higher sensitivity and specificity than IHC7. Furthermore, EBV-encoded RNA (EBER) detection by CISH is considered the gold standard for verifying the presence of EBV in the nuclei of tumor cells, which cannot be determined using PCR because it does not indicate the origin of the infected cell. In addition, the CISH method amplifies the EBER expression signal, making it somewhat more sensitive than the PCR for detecting EBV in samples with low EBER expression8.
The importance of clinical and histological characteristics of EBV infection in the NPC prognosis remains controversial. In addition, limited data are available on the prevalence of EBV infection in Vietnamese patients with NPC and its relationship with specific clinical and histological characteristics. Therefore, this study examined patients with NPC at Military Hospital 103 in Vietnam to clarify these issues.
Methods
Patient cohort and sample size justification
Inclusion criteria
We included patients newly diagnosed with NPC who presented to the Department of Pathology and Forensic Medicine at Military Hospital 103 between October 2020 and October 2021. Patients were randomly selected to participate in this study.
Exclusion criteria
Patients were excluded if they had a history of prior radiation therapy or treatment for NPC, disease recurrence, or a diagnosis of cancers other than NPC.
Thirty-one patients with NPC met the inclusion/exclusion criteria and were enrolled in this study.
The sample size was determined based on the available resources and feasibility of data collection during the study period while considering the statistical power required for the analysis. We also consulted previous studies in the field to ensure that the sample size was appropriate to address the research questions. All procedures performed in this study followed the National Research Committee’s ethical standards and the 1964 Helsinki Declaration and were approved by The Ethics Committee of Military Hospital 103 (approval number: 140/2016/IRB-MH103).
Patient characteristics data
We collected patients’ age, sex, lymph node metastasis, tissue invasion, and metastasis. Their clinical NPC stage was determined according to the eighth edition of the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) Staging System9. NPC was histologically classified based on the WHO 2017 guidelines, which classify NPC tumors into three groups: keratinizing squamous cell carcinoma (type I), differentiated non-keratinizing carcinoma (type II), and undifferentiated carcinoma (type III)3.
Tissue specimens and EBER detection by CISH
The tissue samples were fixed in 10% neutral buffered formalin, processed, and embedded in paraffin blocks. Sections were cut to 5 μm thickness and stained with hematoxylin and eosin (H&E) to evaluate morphology and histology. EBER expression in the specimens was determined by CISH using the ZytoFast EBV Probe Kit (Prod. No.: T-1063-40; Zytovision, Germany). According to the manufacturer, this kit has an analytical sensitivity of 100% and specificity of 100% for detecting EBERs. The CISH procedure was performed according to the manufacturer’s standardized protocol, including deparaffinization, rehydration, pepsin digestion, heat pre-treatment, hybridization with EBER probes, and detection of positive reactivity for EBER expression using mouse-anti-DIG, anti-mouse-HRP-polymer, and diaminobenzidine chromogen solutions. A brown dot in the cell nucleus indicated a positive EBER reaction, while the absence of a color reaction was considered a negative EBER reaction.
Statistical analysis
The statistical analyses were performed using SPSS software (version 22.0; IBM, Inc., NY, USA). Categorical variables are presented as frequencies and percentages. The Chi-square and Fisher’s exact tests were used to evaluate the relationship between EBER expression status and clinicopathological characteristics. Graphs were plotted using GraphPad Prism 9 (GraphPad Software, Inc.). A p-value < 0.05 was considered statistically significant.
| Characteristics | n (%) | |
|---|---|---|
| Age | ||
| < 20 | 1 (3.23) | |
| 20 - 40 | 5 (16.13) | |
| 41 - 60 | 14 (45.16) | |
| > 60 | 11 (35.48) | |
| Sex | ||
| Male | 27 (87.10) | |
| Female | 4 (12.9) | |
| Lymph node metastasis | ||
| Positive | 25 (80.65) | |
| Negative | 6 (19.35) | |
| Tissue Invasion and Metastasis | ||
| Positive | 12 (38.71) | |
| Negative | 19 (61.29) | |
| Clinical tage | ||
| I-II | 10 (32.26) | |
| III-IV | 21 (67.74) | |
| Pathology type/WHO classification | ||
| Type I | 9 (29.03) | |
| Type II | 7 (22.58) | |
| Type III | 15 (48.39) | |
| Characteristic | EBER, n (%) | p | |
|---|---|---|---|
| (+) | (-) | ||
| Age | |||
| < 20 | 1 (100.00) | 0 (0) | 0.0362 |
| 20 - 40 | 5 (100.00) | 0 (0) | |
| 41 - 60 | 6 (42.86) | 8 (57.14) | |
| > 60 | 3 (27.27) | 8 (72.73) | |
| Sex | |||
| Male | 13 (48.15) | 14 (51.85) | 0.9449 |
| Female | 2 (50.00) | 2 (50.00) | |
| Lymph node metastasis | |||
| Positive | 14 (56.00) | 11 (44.00) | 0.0834 |
| Negative | 1 (16.67) | 5 (83.33) | |
| Tissue Invasion and Metastasis | |||
| Positive | 5 (41.67) | 7 (58.33) | 0.5518 |
| Negative | 10 (52.63) | 9 (47.37) | |
| Clinical tage | |||
| I-II | 6 (60.00) | 4 (40.00) | 0.3719 |
| III-IV | 9 (42.86) | 12 (57.14) | |
| Pathology type/WHO classification | |||
| Type I | 0 (0) | 9 (100.00) | 0.0007 |
| Type II | 3 (42.86) | 4 (57.14) | |
| Type III | 12 (80.00) | 3 (20.00) | |
Results
Patients’ clinicopathologic characteristics and EBER expression
To clarify the clinicopathologic characteristics of patients with NPC, we calculated the percentage of some clinical features and histology classifications (Table 1). The patients were aged 19 to 74 years, with most aged >40 years (80.64%); 45.16% were aged 41–60 years. More patients were male (87.10%) than female (12.90%), resulting in a male-to-female ratio of 6.75:1. Therefore, NPC occurs mainly in males and those of middle age and older. Lymph node metastasis was detected in 80.65% of patients with NPC. Tissue invasion and metastasis were detected in 38.71% of patients with NPC. Moreover, 67.74% of patients with NPC were in the late stage (III or IV). These results suggest that patients with NPC often present with advanced disease characterized by lymph node metastasis.
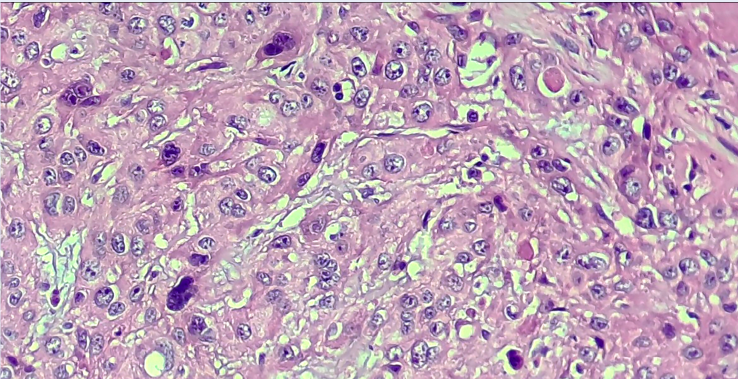
We classified sample histology according to the WHO 2017 classification. The most frequent classification was undifferentiated carcinoma (type III; 48.39%), followed by keratinizing squamous cell carcinoma (type I; 29.03%) and differentiated non-keratinizing carcinoma (type II; 22.58%). Therefore, type III is the most common classification in our study population. The histopathological morphology of the study samples according to the WHO 2017 classification is illustrated in Figure 1.
We used the CISH technique to identify samples from patients with NPC expressing EBER to determine EBV infection. The nuclei of cancer cells but not normal cells stained positive for EBER (Figure 2), indicating that only cancer cells were infected with EBV. Among patients, 48.39% stained positive for EBER, and 51.61% stained negative for EBER (Figure 3).
The relationship between patients’ EBER expression and clinical features
We compared some clinical features, including histology classification, between EBER-positive and -negative patients to evaluate their relationship with EBER expression (Table 2). We found a significant relationship between EBER expression and patient age (p = 0.0362). While EBV infection occurred at all ages, it was more common in younger patients, gradually decreasing with age. However, EBER expression was not significantly associated with other clinical features, such as sex, lymph node metastasis, tissue invasion and metastasis, and cancer stage (p > 0.05).
In addition, EBER expression was significantly associated with histological classification (p = 0.0007). EBER expression was most frequent in undifferentiated carcinoma (type III; 80.00%), followed by differentiated non-keratinizing carcinoma (type II; 42.86%). However, it was not detected in squamous cell carcinoma (type I; Figure 4). Using the CISH method, EBER staining was strongly positive in cancer cells from type II and III NPC but negative in cancer cells from type I NPC (Figure 5), suggesting that EBER may only be expressed in cancer cells from type II and type III but not type I NPC.
Discussion
Previous studies have reported that while NPC can develop at any age, it is most commonly diagnosed between 30 and 50 years. Most patients with NPC in our study were aged 41–60 years (45.16%), consistent with previous reports by Wang et al. (45.6 years) in Taiwan10, Saikia et al. (51.0%) in India11, Lee et al. (53 years) in Hong Kong12, and Zhu et al. (40–59 years) in the United States13. These data suggest certain middle age groups are highly vulnerable to risk factors such as EBV infection, likely due to occupational and environmental exposures.
Most studies have reported a higher NPC incidence in males than females. Globally, the age-standardized incidence of NPC is 2.2 in males and 0.8 in females14, 15. In our study, 87.10% of the patients with NPC were male, and 12.90% were female. Saikia et al. reported that their study sample comprised 70.6% males and 29.4% females11, and Peng et al. reported that their study sample comprised 72.5% males and 27.5% females16. The sex discrepancy may reflect behavioral and occupational variations since males have higher exposures to risk factors such as smoking, alcohol, and industrial carcinogens.
The symptoms of cervical lymph node metastasis and nosebleeds are the most common reasons patients with NPC visit the hospital. We found a high rate (80.65%) of cervical lymph node metastasis in our study sample, consistent with Muchiri et al. (80.0%)17 and Lee et al. (76.9%)12. These findings indicate that lymph node metastasis is a typical symptom of patients with NPC, identifying it as a hallmark of NPC at diagnosis.
Our study found that 67.74% of the patients had late-stage (III or IV) NPC, while 32.26% had earlier-stage (I or II) NPC according to the TNM classification. Al Rajhi et al. reported that all their patients were in stage III-IV18, and Zhu et al. reported that 74.5% of their patients were in stage III-IV (13). The late arrival of patients at the hospital may be due to their disease often progressing silently (asymptomatic) and a lack of knowledge. Our study also found that type III was the most common WHO 2017 classification of NPC (48.39%), consistent with other studies in which type II and III usually predominated; type II and III accounted for 99.4% of NPCs in Peng et al.16 and 100% in Saikia et al.11. In endemic areas such as Southeast Asia and South China19, type III is the most common and aggressive type of NPC, characterized by silent development and late-stage diagnosis with tissue invasion and regional lymph node metastasis, indicating a poor prognosis. The association of type III with poor prognosis underscores the urgency of early NPC identification in high-risk groups.
We used the CISH technique to determine the rate of EBV infection in patients with NPC, finding that 48.39% of patients with NPC were infected with EBV, similar to Saikia et al. (59%)11. However, other studies have reported a higher EBV infection rate using the same method, such as Zeng et al. (71.1% of patients with NPC showed EBER-1 expression)20 and Mäkitie et al. (81% of patients with NPC showed EBER expression)21. The difference in EBV infection rate between studies is likely due to geographical, social-cultural, and environmental factors.
Our study found a significant negative relationship between EBV infection and age. These results are consistent with Macmahon et al., who reported that EBV infection occurs at a young age in most endemic NPC areas22. However, other studies have shown no association between EBV infection and age in patients with NPC11, 20. The variation in EBV infection status and age in patients with NPC between studies could be due to differences in environmental, geographical, and socioeconomic factors. In developing countries, EBV infection typically occurs during childhood and is asymptomatic, whereas in developed countries, EBV infection typically occurs in adolescence or adulthood11. The small sample sizes of patients aged <20 and 20–40 years limit our ability to draw definitive conclusions about EBV infection rates in young patients.
Our study found no significant association between EBER expression status and lymph node metastasis, tissue invasion and metastasis, or clinical stage. The relationship between EBER expression and clinical NPC manifestations is complex and varies among studies. Studies have reported that NPC lesions may or may not depend on the EBV infection (i.e., EBV-positive or negative status). Our findings are consistent with Saikia et al.11 and Nilsson et al., who reported no difference in clinical stages between EBV-positive and EBV-negative patients23. Similarly, Stenmark et al. found no association between viral infection and tumor stage24. However, other studies, such as Ruuskanen et al., demonstrated that EBV-positive tumors had a lower T-stage than EBV-negative ones25. Similarly, Peng et al. reported that EBV-positive patients had significantly higher proportions of lymph node metastasis, tissue invasion, and metastasis than EBV-negative patients16. Therefore, EBER expression is not a decisive factor in clinical manifestations such as lymph node metastasis, tissue invasion and metastasis, or clinical NPC stage.
We found a significant relationship between EBER expression and histological HPC subtypes (p = 0.0007). In our study, 80% of type III NPCs expressed EBER, while 42.86% of type II NPCs but no type I NPCs expressed EBER. Our results are consistent with Tsai et al., who reported high EBER expression in cancer cells from type II and III NPCs but low expression in cancer cells from type I NPCs26. Similarly, Niedobitek et al. found a strong association between EBV infection and non-keratinizing carcinoma (types II and III)27. Other studies have also shown that EBV infection is closely associated with type III28, especially in Southern China and Southeast Asia29, 30. The properties of undifferentiated cancer cells may provide a favorable environment for EBV infection. However, inflammatory and cytokine-rich histiocytosis may also be essential for EBV infecting cancer cells.
Our study had some limitations. First, it was conducted in a single facility with a small sample size of 31 patients. Therefore, it may not be representative of the NPC population in Vietnam. Second, its analytical evaluation was not comprehensive, potentially limiting the interpretation of our findings. Further studies with larger sample sizes and more comprehensive analytical evaluations are needed to confirm our results and explore the relationship between EBV infection and NPC in more detail.
Conclusions
Our study provides important insights into the epidemiology and clinical characteristics of NPC in Vietnam. The age and sex distributions and NPC symptoms, clinical stages, and pathological types in our study were similar to those reported in other regions, suggesting that certain environmental and behavioral factors may contribute to NPC development. Our study also found that EBV infection is a risk factor for NPC in Vietnam and is significantly associated with younger patient age and undifferentiated carcinoma (type III), the most essential and malignant NPC type. Therefore, screening for EBV infection in young, asymptomatic, or high-risk subjects can aid in early NPC diagnosis, management, and prognosis. Overall, our findings contribute to the increasing knowledge of NPC epidemiology and provide valuable information for future research and clinical practice.
Abbreviations
CISH: Chromosomal in situ hybridization; DAB: Diaminobenzidine chromogen; EBV: Epstein-Barr virus; EBER: EBV-encoded RNA; GLOBOCAN: Global Cancer Incidence, Mortality And Prevalence; IBM: International Business Machines Corporation; IHC: Immunohistochemical; NC: Nasopharyngeal carcinoma; NY: New York; PCR: Polymerase chain reaction; RNA: Ribonucleic Acid; SPSS: Statistical Package for the Social Sciences; UICC/AJCC: Union for International Cancer Control/American Joint Committee on Cancer; USA: United States of America; WHO: World Health Organization
Acknowledgments
We thank the patients for joining the study and gratefully acknowledge the support for techniques from Pham Van Thi.
Author’s contributions
Conceived and designed the study: TND PVT DTC. Patient characteristics data and sample collection: TND PVT NMH NTL PVT DTT TDT LTT NMH DTC. Analyzed and interpreted the data: TND PVT NTL LTT DTC. Contributed to drafting and writing the article: TND PVT DTC. Involved in all aspects of the work through correspondence: TND PVT DTC. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All procedures performed in this study followed the national research committee's ethical standards and the 1964 Helsinki declaration, which was also approved by The Ethics Committee of Military hospital 103 (code: 140/2016/IRB-MH103).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Carioli
G.,
Negri
E.,
Kawakita
D.,
Garavello
W.,
La Vecchia
C.,
Malvezzi
M.,
Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: focus on low-risk areas. International Journal of Cancer.
2017;
140
(10)
:
2256-64
.
View Article PubMed Google Scholar -
Globocan. Vietnam - The Global Cancer Observatory 2020 [Available from: http://gco.iarc.fr/today/home.. 2020
.
-
Stelow
E.B.,
Wenig
B.M.,
Update From The 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Nasopharynx. Head and neck pathology.
2017;
11
(1)
:
16-22
.
View Article Google Scholar -
Vaughan
T.L.,
Shapiro
J.A.,
Burt
R.D.,
Swanson
G.M.,
Berwick
M.,
Lynch
C.F.,
Nasopharyngeal cancer in a low-risk population: defining risk factors by histological type. Cancer Epidemiology, Biomarkers & Prevention.
1996;
5
(8)
:
587-93
.
PubMed Google Scholar -
Thompson
L.D.,
Update on nasopharyngeal carcinoma. Head and Neck Pathology.
2007;
1
(1)
:
81-6
.
View Article PubMed Google Scholar -
Chua
M.L.,
Wee
J.T.,
Hui
E.P.,
Chan
A.T.,
Nasopharyngeal carcinoma. Lancet.
2016;
387
(10022)
:
1012-24
.
View Article PubMed Google Scholar -
Nonogaki
S.,
Shirata
N.,
Kimura
L.,
Guerra
J.,
Medeiros
R.,
Paes
R.,
Comparative study of five commercial probes for the detection of Epstein-Barr virus (EBV) by in situ hybridization in cases of nodular sclerosis Hodgki's lymphoma. Jornal Brasileiro de Patologia e Medicina Laboratorial.
2016;
52
:
416-25
.
View Article Google Scholar -
Tsai
S.T.,
Jin
Y.T.,
Mann
R.B.,
Ambinder
R.F.,
Epstein-Barr virus detection in nasopharyngeal tissues of patients with suspected nasopharyngeal carcinoma. Cancer.
1998;
82
(8)
:
1449-53
.
View Article PubMed Google Scholar -
Tang
L.L.,
Chen
Y.P.,
Mao
Y.P.,
Wang
Z.X.,
Guo
R.,
Chen
L.,
Validation of the 8th Edition of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma From Endemic Areas in the Intensity-Modulated Radiotherapy Era. Journal of the National Comprehensive Cancer Network.
2017;
15
(7)
:
913-919
.
View Article Google Scholar -
Wang
W.Y.,
Twu
C.W.,
Chen
H.H.,
Jiang
R.S.,
Wu
C.T.,
Liang
K.L.,
Long-term survival analysis of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA levels. Cancer.
2013;
119
(5)
:
963-70
.
View Article PubMed Google Scholar -
Saikia
A.,
Raphael
V.,
Shunyu
N.B.,
Khonglah
Y.,
Mishra
J.,
Jitani
A.K.,
Analysis of Epstein Barr Virus Encoded RNA Expression in Nasopharyngeal Carcinoma in North-Eastern India: A Chromogenic in Situ Hybridization Based Study. Iranian Journal of Otorhinolaryngology.
2016;
28
(87)
:
267-74
.
PubMed Google Scholar -
Lee
V.H.,
Kwong
D.L.,
Leung
T.W.,
Choi
C.W.,
Lai
V.,
Ng
L.,
Prognostication of serial post-intensity-modulated radiation therapy undetectable plasma EBV DNA for nasopharyngeal carcinoma. Oncotarget.
2017;
8
(3)
:
5292-308
.
View Article PubMed Google Scholar -
Zhu
Y.,
Song
X.,
Li
R.,
Quan
H.,
Yan
L.,
Assessment of Nasopharyngeal Cancer in Young Patients Aged below 30 Years. Frontiers in Oncology.
2019;
9
:
1179
.
View Article PubMed Google Scholar -
Althubiti
M.A.,
Nour Eldein
M.M.,
Trends in the incidence and mortality of cancer in Saudi Arabia. Saudi Medical Journal.
2018;
39
(12)
:
1259-62
.
View Article PubMed Google Scholar -
Chen
Y.P.,
Chan
A.T.,
Le
Q.T.,
Blanchard
P.,
Sun
Y.,
Ma
J.,
Nasopharyngeal carcinoma. Lancet.
2019;
394
(10192)
:
64-80
.
View Article PubMed Google Scholar -
Peng
H.,
Chen
L.,
Zhang
Y.,
Guo
R.,
Li
W.F.,
Mao
Y.P.,
Survival analysis of patients with advanced-stage nasopharyngeal carcinoma according to the Epstein-Barr virus status. Oncotarget.
2016;
7
(17)
:
24208-16
.
View Article PubMed Google Scholar -
Muchiri
M.,
Demographic study of nasopharyngeal carcinoma in a hospital setting. East African Medical Journal.
2008;
85
(8)
:
406-11
.
View Article PubMed Google Scholar -
Rajhi
N. Al,
Sebaie
M. El,
Khafaga
Y.,
Zahrani
A. Al,
Mohamed
G.,
Amro
A. Al,
Nasopharyngeal carcinoma in Saudi Arabia: clinical presentation and diagnostic delay. EMHJ-Eastern Mediterranean Health Journal.
2009;
15
(5)
:
1301-1307
.
-
Sharif
S.E.,
Zawawi
N.,
Yajid
A.I.,
Shukri
N.M.,
Mohamad
I.,
Pathology classification of nasopharyngeal carcinoma. In: Abdullah B, Balasubramanian A, Lazim NM, editors. An Evidence-Based Approach to the Management of Nasopharyngeal Cancer: Academic Press; 2020. p. 73-92.. 2020
.
-
Zeng
Z.,
Fan
S.,
Zhang
X.,
Li
S.,
Zhou
M.,
Xiong
W.,
Epstein-Barr virus-encoded small RNA 1 (EBER-1) could predict good prognosis in nasopharyngeal carcinoma. Clinical & Translational Oncology.
2016;
18
(2)
:
206-11
.
View Article PubMed Google Scholar -
Mäkitie
A.A.,
MacMillan
C.,
Ho
J.,
Shi
W.,
Lee
A.,
O'Sullivan
B.,
Loss of p16 expression has prognostic significance in human nasopharyngeal carcinoma. Clinical Cancer Research.
2003;
9
(6)
:
2177-84
.
PubMed Google Scholar -
MacMahon
B.,
Epidemiology of Hodgkin's disease. Cancer Research.
1966;
26
(6)
:
1189-201
.
PubMed Google Scholar -
Nilsson
J.S.,
Forslund
O.,
Andersson
F.C.,
Lindstedt
M.,
Greiff
L.,
Intralesional EBV-DNA load as marker of prognosis for nasopharyngeal cancer. Scientific Reports.
2019;
9
(1)
:
15432
.
View Article PubMed Google Scholar -
Stenmark
M.H.,
McHugh
J.B.,
Schipper
M.,
Walline
H.M.,
Komarck
C.,
Feng
F.Y.,
Nonendemic HPV-positive nasopharyngeal carcinoma: association with poor prognosis. International Journal of Radiation Oncology, Biology, Physics.
2014;
88
(3)
:
580-8
.
View Article PubMed Google Scholar -
Ruuskanen
M.,
Irjala
H.,
Minn
H.,
Vahlberg
T.,
Randen-Brady
R.,
Hagström
J.,
Epstein-Barr virus and human papillomaviruses as favorable prognostic factors in nasopharyngeal carcinoma: A nationwide study in Finland. Head & Neck.
2019;
41
(2)
:
349-57
.
View Article PubMed Google Scholar -
Tsai
S.T.,
Jin
Y.T.,
Su
I.J.,
Expression of EBER1 in primary and metastatic nasopharyngeal carcinoma tissues using in situ hybridization. A correlation with WHO histologic subtypes. Cancer.
1996;
77
(2)
:
231-6
.
View Article PubMed Google Scholar -
Niedobitek
G.,
Hansmann
M.L.,
Herbst
H.,
Young
L.S.,
Dienemann
D.,
Hartmann
C.A.,
Epstein-Barr virus and carcinomas: undifferentiated carcinomas but not squamous cell carcinomas of the nasopharynx are regularly associated with the virus. The Journal of Pathology.
1991;
165
(1)
:
17-24
.
View Article PubMed Google Scholar -
Adham
M.,
Kurniawan
A.N.,
Muhtadi
A.I.,
Roezin
A.,
Hermani
B.,
Gondhowiardjo
S.,
Nasopharyngeal carcinoma in Indonesia: epidemiology, incidence, signs, and symptoms at presentation. Chinese Journal of Cancer.
2012;
31
(4)
:
185-96
.
View Article PubMed Google Scholar -
Lo
K.W.,
To
K.F.,
Huang
D.P.,
Focus on nasopharyngeal carcinoma. Cancer Cell.
2004;
5
(5)
:
423-8
.
View Article PubMed Google Scholar -
Tsao
S.W.,
Yip
Y.L.,
Tsang
C.M.,
Pang
P.S.,
Lau
V.M.,
Zhang
G.,
Etiological factors of nasopharyngeal carcinoma. Oral Oncology.
2014;
50
(5)
:
330-8
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 9 (2023)
Page No.: 5924-5933
Published on: 2023-09-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 4053 times
- PDF downloaded - 1070 times
- XML downloaded - 114 times
 Biomedpress
Biomedpress







