Abstract
Background: Given the established effectiveness of gabapentin and NSAIDs in reducing postoperative pain and the paucity of comparative studies of gabapentin and NSAIDs for postoperative pain management, our current study was designed to assess and compare the effectiveness of celecoxib and gabapentin in preventing post-cesarean section pain.
Methods: This randomized clinical trial was conducted on pregnant women who underwent elective caesarean sections with spinal anesthesia who were referred to Mousavi Hospital, Iran, in 2022. Patients meeting inclusion criteria were grouped via balanced block randomization into three groups. Group 1 received 600 mg of gabapentin and group 2 received 200 mg of celecoxib 1 hour before the surgery, while group 3 did not receive any pain-relief medication before the surgery. Patients’ pain levels were recorded using the visual analog scale (VAS) in the recovery room, as well as at 1, 6, 12, and 24 hours after surgery. Comparisons between groups were made using one-way ANOVA and chi-squared tests.
Results: The mean age of participating mothers was 27.4 ± 4.69 years. There were no statistically significant differences in the mean nausea levels or the request for additional medication among the groups at any time points (0, 1, 6, 12, and 24 hours after the intervention) in mothers following cesarean section surgery (p > 0.05). At all time points, the mean VAS score in the gabapentin group was lower than in the celecoxib and control groups (p < 0.05).
Conclusion: Our study suggests that preoperative administration of 600 mg of gabapentin can effectively reduce postoperative pain in women undergoing elective cesarean section surgery. No significant differences in side effects were observed among groups. This research highlights the potential of gabapentin as a valuable component of pain management strategies for cesarean section patients.
Introduction
Pain following a cesarean section is a substantial source of concern for numerous women. During this period, the mother not only has to endure the pain resulting from the cesarean section but also must be capable of caring for the newborn, who requires special attention1. Furthermore, achieving effective pain relief following a cesarean section is of paramount importance given the heightened risk of thromboembolic conditions that can exacerbate due to the immobility resulting from postpartum pain2. The following consequences of these complications include various economic and medical concerns, such as prolonged hospitalization, the need for re-hospitalization, increased patient recovery costs, and ultimately patient dissatisfaction with hospital care3.
Traditional approaches to managing acute postoperative pain primarily involve the administration of oral or injectable analgesic medications on an as-needed basis. Narcotic medications, especially in injectable form, are commonly utilized to alleviate acute pain. However, postoperative pain is a complex phenomenon that cannot be effectively controlled by single-agent narcotic therapy alone. Furthermore, the use of narcotics is associated with dose-dependent side effects, including respiratory depression, nausea, vomiting, urinary retention, itching, sedation, and postoperative ileus. Therefore, it appears reasonable and rational to administer substances that can enhance the analgesic effects of narcotics, ultimately leading to improved pain relief with reduced opioid consumption4, 5.
Chronic postoperative pain often has a neuropathic component. Neuropathic pain can even be observed in the early stages after surgery. For this reason, drugs traditionally used to treat chronic neuropathic pain are increasingly being used as adjunctive therapy for postoperative pain6. These drugs include antidepressants like amitriptyline, anticonvulsants like gabapentinoids, N-methyl-D-aspartate (NMDA) receptor antagonists like ketamine and magnesium, membrane stabilizers like lidocaine, and alpha-2 agonists like clonidine. Recent studies have demonstrated that drugs like gabapentin and pregabalin can not only alleviate the intensity of acute postoperative pain and reduce the need for opioids but also can contribute to the prevention of chronic postoperative pain7.
The most recognized mechanism for the analgesic effects of gabapentinoids is their binding to the α2δ-1 subunit of voltage-gated calcium channels at presynaptic synapses and the modulation of neurotransmitter release, particularly of glutamate, which reduces neuronal excitability and central sensitization, leading to hypoalgesia and allodynia8. Recent studies have demonstrated that these drugs disrupt the transfer of α2δ-1 subunits to the terminals of dorsal root ganglion (DRG) neurons, reducing calcium influx into the cell. Nonetheless, alternative mechanisms have been suggested for the action of these drugs, which involve the activation of inhibitory pathways within the noradrenergic pain system in the spinal cord and brain, heightened activity of voltage-gated potassium channels, and impacts on NMDA receptors. However, the precise degree to which these mechanisms play a role in the analgesic effects of these drugs remains incompletely understood9, 10. Another group of drugs used to reduce postoperative pain and mitigate the side effects associated with opioid use includes NSAIDs. This drug class inhibits the enzyme cyclooxygenase, which prevents the production of prostaglandins that cause inflammation, fever, and pain11.
Considering the results of this previous research on the beneficial effects of gabapentin and NSAIDs in managing post-cesarean section pain, as well as the limited number of studies in this area and the significant side effects, particularly the risk of respiratory depression, associated with conventional analgesic drugs frequently used during cesarean sections, it becomes imperative to explore alternative and more suitable options for pain relief in these patients. Given the established effectiveness of gabapentin and NSAIDs in reducing postoperative pain and the paucity of comparative studies of gabapentin and NSAIDs for postoperative pain management, our current study was designed to assess and compare the effectiveness of celecoxib and gabapentin in preventing post-cesarean section pain.
Methods
The present study was a randomized controlled clinical trial conducted in 2022 at Ayatollah Mousavi Hospital in Zanjan, Iran, with the aim of comparing the effectiveness of celecoxib and gabapentin in reducing post-cesarean section pain in pregnant women.
The inclusion criteria for study participation were as follows: patient’s voluntary willingness to participate, eligibility for non-emergency cesarean section surgery, absence of allergies to gabapentin and NSAIDs, age between 20 and 45 years, lack of contraindications for spinal anesthesia (e.g., patient dissatisfaction and coagulation disorders), lack of infection at the injection site, lack of history of chronic pain or neurological or psychiatric disorders, and lack of use of any pain-relief medication in the 24 hours prior to the study. Exclusion criteria included patient withdrawal from the study at any point, cesarean section duration exceeding 2 hours, an increase in incision length for any reason, occurrence of unusual complications during surgery, conversion from spinal anesthesia to general anesthesia for any reason, and receiving pain-relief medication during the surgery.
The study protocol received approval from the ethics committee of Zanjan University of Medical Sciences under the code IR.ZUMS.REC.1401.051. Additionally, the study was registered in the Iranian Registry of Clinical Trials (IRCT) with the code IRCT20220517054889N1. Patients meeting the inclusion criteria were selected as a convenience sample. They were first classified into three groups using balanced block randomization for intervention assignment:
Prior to the commencement of surgery, patients received an explanation of the visual analog scale (VAS) for pain assessment. On this scale, 0 signifies the absence of pain, while 10 denotes the most severe pain. Patients’ pain levels were documented using this scale in the recovery room, as well as at 1, 6, 12, and 24 hours post-surgery. Any potential side effects stemming from the medications, such as seizures, reduced level of consciousness, nausea, and vomiting, were likewise recorded during the 24 hours following the surgical procedure.
At any time following the cesarean section when a patient reported pain on the VAS greater than or equal to 4, an additional dose of diclofenac suppository (100 mg) was administered. The cumulative quantity of supplementary pain-relief medication administered to patients within a 24-hour period was calculated and documented.
Data were collected using a researcher-designed checklist that included demographic and clinical variables such as age, history of previous pregnancies, underlying diseases, and pregnancy-related illnesses.
To compare the means of each of the examined variables before and after the intervention among the three groups, ANOVA was employed. When variables failed to meet the assumption of normality, Kruskal–Wallis nonparametric tests were used instead. To compare qualitative variables among the three groups, chi-square tests were employed. In the final analysis, a repeated measures ANOVA was used to compare the mean pain intensity at different time points among the three groups. The data were analyzed using SPSS software, version 24, with a significance level set at p < 0.05.
Results
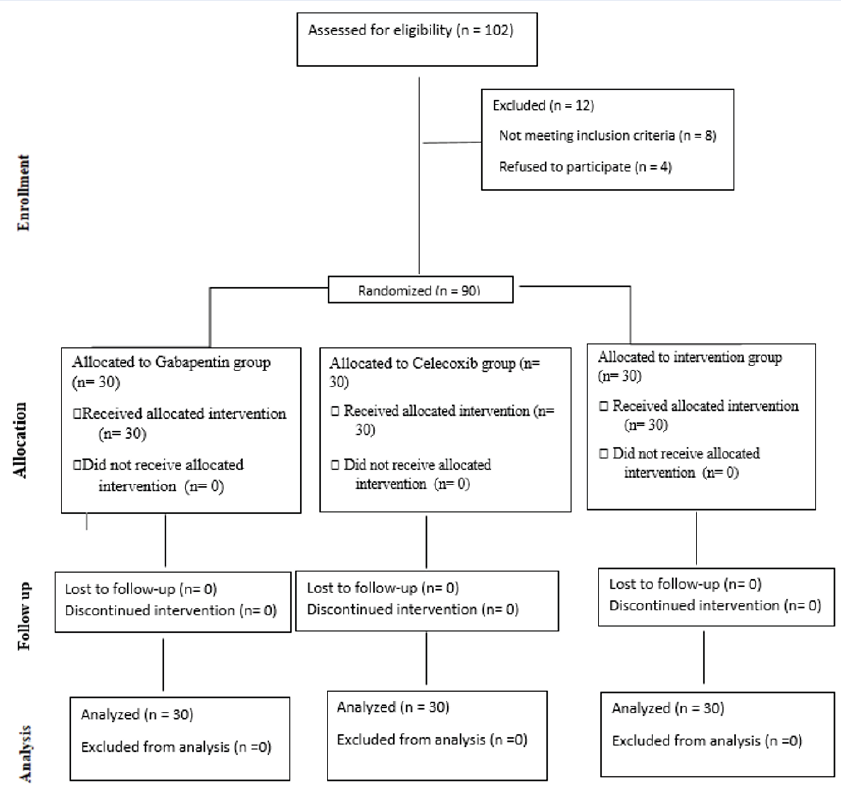
Of a total of 102 candidates for participation in the research, 8 individuals did not meet the entry criteria, and 4 participants declined to participate in the study. Ultimately, analysis was conducted with data from 90 individuals. Figure 1 illustrates a flowchart describing the clinical trial.
| Variable | Gabapentin group (n = 30) | Celecoxib group (n = 30) | Control group (n = 30) | P value* |
|---|---|---|---|---|
| Mother’s weight (kg) (Mean ± SD) | 83.8 ± 8.64 | 83.11 ± 11.3 | 83.99 ± 9.23 | 0.963 a |
| Neonatal ‘s weight (kg) (Mean ± SD) | 3.37 ± 0.71 | 3.46 ± 0.63 | 3.61 ± 0.56 | 0.325 a |
| Age (years) (Mean ± SD) | 28.1 ± 4.42 | 27.77 ± 4.59 | 27.87 ± 5.2 | 0.962 a |
| Apgar (Mean ± SD) | 8.0 ± 0.69 | 7.93 ± 0.69 | 7.90 ± 0.66 | 0.848 a |
| Control group (n = 30) | Celecoxib group (n = 30) | Gabapentin group (n = 30) | Total | p-value | ||
|---|---|---|---|---|---|---|
| Sleepiness | n | 1 | 1 | 0 | 2 | 0.600 |
| Frequency | 3.3% | 3.3% | 0% | 2.2% |
| Time | Gabapentin group (n = 30) | Gabapentin group (n = 30) | Control group (n = 30) | Total | p-value |
|---|---|---|---|---|---|
| Recovery, n (%) | 15 (50%) | 17 (56.7%) | 16 (53.3%) | 48 (53.3%) | 0.875 |
| 1 hour later, n (%) | 22 (73.3%) | 17 (56.7%) | 19 (63.3%) | 58 (64.4%) | 0.398 |
| 6 hour later, n (%) | 6 (20%) | 7 (23.3%) | 8 (26.7%) | 21 (23.3%) | 0.830 |
| 12 hour later, n (%) | 5 (16.7%) | 4 (13.3%) | 6 (20.07%) | 15 (16.7) | 0.787 |
| 24 hour later, n (%) | 3 (10%) | 2 (6.7%) | 3 (10%) | 8 (8.9%) | 0.872 |
| Time | Gabapentin group (n = 30) | Celecoxib group (n = 30) | Control group (n = 30) | Total | p-value |
|---|---|---|---|---|---|
| Recovery, n (%) | 13 (53.3%) | 14 (46.7%) | 15 (50%) | 42 (46.7%) | 0.875 |
| 1 hour later, n (%) | 11 (36.7%) | 14 (46.7%) | 14 (46.7%) | 39 (43.3%) | 0.875 |
| 6 hour later, n (%) | 18 (60%) | 19 (63.3%) | 19 (63.3%) | 56 (62.2%) | 0.954 |
| 12 hour later, n (%) | 19 (63.3%) | 18 (62.1%) | 19 (65.5%) | 56 (63.6) | 0.963 |
| 24 hour later, n (%) | 11 (36.7%) | 12 (40%) | 13 (43.3%) | 36 (40%) | 0.875 |
| Time | Gabapentin group (n = 30) | Celecoxib group (n = 30) | Control group (n = 30) |
|---|---|---|---|
| Recovery, (Mean ± SD) | 3.73 ± 0.36 | 3.9 ± 0.39 | 3.83 ± 0.46 |
| 1 hour later, (Mean ± SD) | 4.3 ± 0.29 | 4.56 ± 0.32 | 5.0 ± 0.28 |
| 6 hour later, (Mean ± SD) | 4.28 ± 0.37 | 5.8 ± 0.35 | 5.8 ± 0.31 |
| 12 hour later, (Mean ± SD) | 4.16 ± 0.33 | 4.5 ± 0.28 | 5.63 ± 0.29 |
| 24 hour later, (Mean ± SD) | 3.37 ± 0.31 | 3.73 ± 0.37 | 4.06 ± 0.32 |
The average age of mothers participating in the study was 27.4 ± 4.69 years. The results of the ANOVA did not show significant differences in the average weights of mothers or their neonates among the study groups (p > 0.05) (Table 1). There was not a significant difference in the Apgar scores of newborns among the groups (p = 0.848).
We did not find a significant difference in the level of maternal sleepiness during the recovery period (time point 0) among the intervention and control groups (p = 0.600) (Table 2).
No statistically significant differences were observed in the mean levels of nausea among the groups at any time points (0, 1, 6, 12, and 24 hours after the intervention) in mothers who underwent cesarean section surgery (p > 0.05) (Table 3).
Furthermore, there was not a significant difference in the request for additional medication among the groups at any of the five time points (p > 0.05) (Table 4).
Statistically significant differences were observed in the overall mean VAS scores; the average VAS score in the gabapentin group was significantly lower than in the other groups (p = 0.048). Significant differences in mean VAS scores were also found among the study groups at various time points (p = 0.005). At all time points, the mean VAS score in the gabapentin group was lower than in the celecoxib and control groups (Table 5). The results of the repeated measures ANOVA comparing the mean VAS scores across different time points among the three study groups demonstrated a significant effect of time point on VAS score (p < 0.001).
Discussion
We found that the administration of 600 mg of gabapentin to women 1 hour before elective cesarean section surgery effectively reduced patients’ pain in the postoperative hours compared to the administration of celecoxib and the control. This finding is consistent with the results of a study conducted by Hafez El Saied Hafez and colleagues12. Various studies have also reported the analgesic effects of gabapentin during different surgical procedures, such as orthognathic surgery13 and uterine curettage14.
Our study results highlight the effectiveness of gabapentin in pain relief following cesarean section surgery. Consistent with our findings, Tiffany and colleagues14 also demonstrated that the administration of gabapentin significantly decreased patient pain in the intervention group in comparison to the control group. Furthermore, the results of a study by Beigom Khezri15 showed that gabapentin, along with vitamin B complex, effectively reduced pain and analgesic consumption during the first 12 hours in women undergoing spinal anesthesia following cesarean section. According to the results of another clinical randomized trial, the administration of 600 and 900 mg of gabapentin effectively led to pain reduction in both groups of women following cesarean section surgery at time points 2, 4, 10, 14, and 18 hours post-operation compared to the control group12. However, the findings of David and colleagues16 did not report a significant impact of gabapentin administration on cesarean section pain reduction. Horne and colleagues in 202017 also did not report any significant effect of gabapentin administration on reducing chronic pelvic pain in women; in addition, gabapentin was associated with side effects. Similarly, Short and colleagues18 did not report any statistically significant differences between groups administered 300 and 600 mg of gabapentin and a control group. Researchers attributed this finding to the potential long-lasting analgesic effects of spinal morphine in all groups, thereby limiting the ability to distinguish the analgesic effects of gabapentin.
The use of preoperative pain management therapies to reduce postoperative pain is referred to as preemptive analgesia12. Effective pain control in mothers before cesarean section is important for various reasons, as inadequate pain relief after cesarean section can affect the mother’s ability to care for and feed the newborn12. Opioid medications effectively reduce pain during surgical procedures; however, their use has limitations and can cause various side effects, making the development of combination drug therapies essential19, 20, 21. Gabapentin, by binding to the α2δ-1 subunit of calcium channels in the dorsal spinal cord, reduces central nervous system excitability, leading to pain reduction22. The results of a meta-analysis have shown that the use of gabapentin, regardless of the type of surgery, results in decreased opioid consumption by patients22. However, an optimal gabapentin dose has not yet been recommended22. In our study, a dose of 600 mg of gabapentin was administered; this dose has been used in various studies during cesarean section surgeries12, 18.
Our study did not report a significant difference in the level of nausea among mothers in the gabapentin, celecoxib, and control groups. This finding contrasts with the results of a study conducted by Alaasar and colleagues23. Additionally, Ghiasy and colleagues24 found the administration of 600 mg of gabapentin prior to surgery to be effective at reducing postoperative nausea in patients undergoing orthoplasty, which differs from our results. Researchers attributed this effectiveness to the indirect effects of the reduced opioid consumption following gabapentin administration. However, the results of Tiffany and colleagues14 did not support a significant effect of gabapentin in reducing patient nausea, aligning with our findings.
Celecoxib functions as a selective COX-2 inhibitor that leads to the inhibition of prostaglandin synthesis in the spinal cord and peripheral nervous system, resulting in pain reduction after surgery25. The results of a study by Joseph and colleagues13 showed that the administration of pre-gabapentin and celecoxib led to a reduction in pain after lower jaw surgery. Additionally, the results of a study by Saito and colleagues25 indicated the effectiveness of celecoxib in achieving pain relief in patients undergoing laparoscopic inguinal hernia repair. In a study by Choubsaz and colleagues26, the addition of celecoxib to a gabapentin regimen significantly reduced patient pain following inguinal hernia repair. In their study, both drugs were used simultaneously. However, in our study, the effects of each drug were examined separately.
In our study, we did not find any statistically significant differences in the request for additional medication or the level of maternal drowsiness among the groups at different time points. However, in a study by Ghiasy and colleagues24, the use of gabapentin was associated with reduced opioid consumption and drowsiness. Alaasar and colleagues23 also found that the administration of 300 mg of pregabalin was more effective at reducing pain and nausea in patients compared to 900 mg of gabapentin. Nevertheless, in our study, gabapentin effectively reduced maternal pain compared to the control.
Our study findings revealed no discernible impact of gabapentin or celecoxib administration on neonatal Apgar scores. Other studies have also reported the administration of gabapentin without side effects and without delays in neonatal discharge or feeding23, 27. Consistent with the results of the current study, an investigation conducted by Short and colleagues18 likewise failed to detect any statistically significant disparities in Apgar scores or arterial cord blood pH in newborns following gabapentin administration. In a study by Tiffany and colleagues14, few side effects were reported following gabapentin consumption. However, a study by Peng and colleagues28 showed that gabapentin administration was associated with dizziness in patients.
While the use of gabapentin as an analgesic in women following cesarean section did not have adverse outcomes for neonates, there is still a need for more evidence-based studies to develop pain management regimens for mothers. This study had limitations, including the use of the VAS, which is a subjective measure and may be influenced by individual and cultural factors of the participants, to assess patient pain. Additionally, the measurement of outcome variables in this study was performed only within the first 24 hours post-surgery. Therefore, future studies with larger sample sizes and assessments of patient pain beyond 24 hours, as well as evaluation of parameters such as patient satisfaction and monitoring of post-discharge complications in mothers, are recommended.
Conclusion
In summary, our study suggests that preoperative administration of 600 mg of gabapentin can effectively reduce postoperative pain in women undergoing elective cesarean section surgery. No substantial disparities in side effects were observed among groups. This research highlights the potential of gabapentin as a valuable component of pain management strategies for cesarean section patients. Further studies with larger sample sizes and extended postoperative assessments are needed to confirm and refine these findings.
Abbreviations
None.
Acknowledgments
Deputy of Research and Technology of Zanjan University of Medical Sciences approved our study and financially supported the study. We would like gratefully acknowledge the medical staff of the Mousvai Hospitol.
Author’s contributions
All authors significantly contributed to this works. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study protocol received approval from the ethics committee of Zanjan University of Medical Sciences under the code IR.ZUMS.REC.1401.051. Additionally, the study was registered in the Iranian Registry of Clinical Trials (IRCT) with the code IRCT20220517054889N1.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Babazade
R.,
Vadhera
R.B.,
Krishnamurthy
P.,
Varma
A.,
Doulatram
G.,
Saade
G.R.,
Acute postcesarean pain is associated with in-hospital exclusive breastfeeding, length of stay and post-partum depression. Journal of Clinical Anesthesia.
2020;
62
:
109697
.
View Article PubMed Google Scholar -
Shang
A.B.,
Gan
T.J.,
Optimising postoperative pain management in the ambulatory patient. Drugs.
2003;
63
(9)
:
855-67
.
View Article PubMed Google Scholar -
Sutton
C.D.,
Carvalho
B.,
Optimal pain management after cesarean delivery. Anesthesiology Clinics.
2017;
35
(1)
:
107-24
.
View Article PubMed Google Scholar -
Mitra
S.,
Carlyle
D.,
Kodumudi
G.,
Kodumudi
V.,
Vadivelu
N.,
New advances in acute postoperative pain management. Current Pain and Headache Reports.
2018;
22
(5)
:
35
.
View Article PubMed Google Scholar -
Luo
J.,
Min
S.,
Postoperative pain management in the postanesthesia care unit: an update. Journal of Pain Research.
2017;
10
:
2687-98
.
View Article PubMed Google Scholar -
Peng
Z.,
Li
H.,
Zhang
C.,
Qian
X.,
Feng
Z.,
Zhu
S.,
A retrospective study of chronic post-surgical pain following thoracic surgery: prevalence, risk factors, incidence of neuropathic component, and impact on qualify of life. PLoS One.
2014;
9
(2)
:
e90014
.
View Article PubMed Google Scholar -
Kumar
A.H.,
Habib
A.S.,
The role of gabapentinoids in acute and chronic pain after surgery. Current Opinion in Anaesthesiology.
2019;
32
(5)
:
629-34
.
View Article PubMed Google Scholar -
Chincholkar
M.,
Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. British Journal of Anaesthesia.
2018;
120
(6)
:
1315-34
.
View Article PubMed Google Scholar -
Kukkar
A.,
Bali
A.,
Singh
N.,
Jaggi
A.S.,
Implications and mechanism of action of gabapentin in neuropathic pain. Archives of Pharmacal Research.
2013;
36
(3)
:
237-51
.
View Article PubMed Google Scholar -
Taylor
C.P.,
Harris
E.W.,
Analgesia with gabapentin and pregabalin may involve N-methyl-d-aspartate receptors, neurexins, and thrombospondins. The Journal of Pharmacology and Experimental Therapeutics.
2020;
374
(1)
:
161-74
.
View Article PubMed Google Scholar -
Abou Zeid
H.,
Kallab
R.,
Najm
M.A.,
Jabbour
H.,
Noun
R.,
Sleilati
F.,
Safety and efficacy of non-steroidal anti-inflammatory drugs (NSAIDs) used for analgesia after bariatric surgery: a retrospective case-control study. Obesity Surgery.
2019;
29
(3)
:
911-6
.
View Article PubMed Google Scholar -
Hafez
M.H.,
Abdelhamid
M.H.,
Youssef
M.M.,
Abdelrahim
I.K.,
Randomized controlled trial of two oral regimens of gabapentin versus placebo in patients for Cesarean section under spinal anesthesia regarding postoperative pain, sedation, nausea and vomiting. Egyptian Journal of Anaesthesia.
2017;
33
(1)
:
59-65
.
View Article Google Scholar -
Cillo
J.E.,
Dattilo
D.J.,
Pre-emptive analgesia with pregabalin and celecoxib decreases postsurgical pain following maxillomandibular advancement surgery: a randomized controlled clinical trial. Journal of Oral and Maxillofacial Surgery.
2014;
72
(10)
:
1909-14
.
View Article PubMed Google Scholar -
Hailstorks
T.P.,
Cordes
S.M.D.,
Cwiak
C.A.,
Gray
B.A.,
Ge
L.,
Moore
R.H.,
Gabapentin as an adjunct to paracervical block for perioperative pain management for first-trimester uterine aspiration: a randomized controlled trial. American Journal of Obstetrics and Gynecology.
2020;
223
(6)
:
884-e1
.
View Article Google Scholar -
Khezri
M.B.,
Nasseh
N.,
Soltanian
G.,
The comparative preemptive analgesic efficacy of addition of vitamin B complex to gabapentin versus gabapentin alone in women undergoing cesarean section under spinal anesthesia: A prospective randomized double-blind study. Medicine.
2017;
96
(15)
:
e6545
.
View Article PubMed Google Scholar -
Monks
D.T.,
Hoppe
D.W.,
Downey
K.,
Shah
V.,
Bernstein
P.,
Carvalho
J.C.,
A Perioperative Course of Gabapentin Does Not Produce a Clinically Meaningful Improvement in Analgesia after Cesarean Delivery: A Randomized Controlled Trial. Anesthesiology.
2015;
123
(2)
:
320-6
.
View Article PubMed Google Scholar -
Horne
A.W.,
Vincent
K.,
Hewitt
C.A.,
Middleton
L.J.,
Koscielniak
M.,
Szubert
W.,
GaPP2 collaborative
Gabapentin for chronic pelvic pain in women (GaPP2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet.
2020;
396
(10255)
:
909-17
.
View Article PubMed Google Scholar -
Short
J.,
Downey
K.,
Bernstein
P.,
Shah
V.,
Carvalho
J.C.,
A single preoperative dose of gabapentin does not improve postcesarean delivery pain management: a randomized, double-blind, placebo-controlled dose-finding trial. Anesthesia and Analgesia.
2012;
115
(6)
:
1336-42
.
View Article PubMed Google Scholar -
Habibi
M.R.,
Masoomi
S.,
Godazandeh
G.A.,
Emadi
S.A.,
Akhbari
P.,
Nooraee
S.M.,
The effect of magnesium sulphate on postoperative pain and opioid consumption in inguinal hernia surgery. Journal of Mazandaran University of Medical Sciences.
2012;
21
(86)
:
168-73
.
-
Norouzi
A.,
Haji-beigi
L.,
Abbasi Talarposhti
M.,
Mashhadi
E.,
Jamilian
M.,
The effect of adding intravenous Metoclopramide to Pethidine for postoperative cesarean section pain. Journal of Arak University of Medical Sciences.
2006;
9
(4)
:
93-9
.
-
Tramer
M.R.,
Schneider
J.,
Marti
R.A.,
Rifat
K.,
Role of magnesium sulfate in postoperative analgesia. Anesthesiology.
1996;
84
(2)
:
340-7
.
View Article PubMed Google Scholar -
Fabritius
M.L.,
Geisler
A.,
Petersen
P.L.,
Wetterslev
J.,
Mathiesen
O.,
Dahl
J.B.,
Gabapentin in procedure-specific postoperative pain management - preplanned subgroup analyses from a systematic review with meta-analyses and trial sequential analyses. BMC Anesthesiology.
2017;
17
(1)
:
85
.
View Article PubMed Google Scholar -
Alaasar
N.M.,
soud
D.E.,
El-Deen
A.M. Galal,
Elfattah
A.I. Abd,
Oral Gabapentin versus Pregabalin for Postoperative Pain Relief in Elective Cesarean Section Patients under Spinal Anesthesia. The Egyptian Journal of Hospital Medicine.
2020;
81
(1)
:
1330-7
.
View Article Google Scholar -
Ghiasy
S.,
Tayebi-Azar
A.,
Alinezhad
A.,
Fallah-Karkan
M.,
Salimi
H.,
Hojjati
S.A.,
The Effect Of Preoperative Gabapentin on Pain Severity After Posterior Urethral Surgery: A Randomized, Double-Blind, Placebo-Controlled Study. Urology Journal.
2020;
17
(6)
:
626-30
.
PubMed Google Scholar -
Saito
T.,
Iwamoto
S.,
Murotani
K.,
Hashimoto
A.,
Kurahashi
S.,
Fukami
Y.,
Efficacy of celecoxib as preemptive analgesia for patients undergoing laparoscopic inguinal hernia repair: a randomized trial. Surgery Today.
2021;
51
(7)
:
1118-25
.
View Article PubMed Google Scholar -
Choubsaz
M.,
Yari
M.,
Amirifard
N.,
Yeganeh
N.,
Rezaei
M.,
Delavardevin
N.,
Effect of adding celecoxib to a single dose of gabapentin on postoperative pain relief. Majallah-i Danishgah-i Ulum-i Pizishki-i Kirmanshah.
2015;
18
(10)
.
-
Pallavi
A.,
Payal
J.,
Effect of Gabapentin Premedication on Post Dural Puncture Headache in Patients Undergoing Elective Cesarean Section. Academia Anesthesiologica International.
2017;
2
(1)
:
35-8
.
-
Türe
H.,
Sayin
M.,
Karlikaya
G.,
Bingol
C.A.,
Aykac
B.,
Türe
U.,
The analgesic effect of gabapentin as a prophylactic anticonvulsant drug on postcraniotomy pain: a prospective randomized study. Anesthesia and Analgesia.
2009;
109
(5)
:
1625-31
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 1 (2024)
Page No.: 6156-6163
Published on: 2024-01-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 2536 times
- PDF downloaded - 833 times
- XML downloaded - 71 times
 Biomedpress
Biomedpress



