Abstract
Introduction: The vascular endothelium plays a pivotal role in maintaining vascular function and physiological balance. The degradation and injury of endothelial cells are critical pathological events in the progression of vascular diseases, leading to cell death. One such cell death mechanism, ferroptosis, is an iron-dependent form of necrosis characterized by extensive lipid peroxidation-mediated membrane damage and the toxic effects of iron and lipid peroxidation. Kaempferol, a flavonoid, is celebrated for its antioxidant, anti-inflammatory, and anti-cancer properties. Despite these benefits, the impact of Kaempferol on endothelial cell ferroptosis and its potential therapeutic applications in vascular diseases have yet to be fully elucidated.
Methods: Cell viability was assessed using the Cell Counting Kit-8 (CCK-8) assay. Oxidative stress and lipid peroxidation were measured using Dihydroethidium (DHE) and C11-BODIPY 581/591, respectively. The protein and RNA levels of ferroptosis-associated molecules, including solute carrier family 7 member 11 (SLC7A11) and glutathione peroxidase 4 (GPX4), were determined through Western blotting and real-time fluorescence quantitative polymerase chain reaction (qPCR).
Results: Treatment with a glutathione peroxidase 4 inhibitor (RSL3) led to rapid cytotoxicity in human umbilical vein endothelial cells (HUVECs). Notably, Kaempferol demonstrated a significant protective effect against RSL3-induced ferroptosis in HUVECs. Kaempferol treatment reduced the accumulation of reactive oxygen species (ROS) and exhibited distinctive morphological changes associated with ferroptosis. Moreover, Kaempferol treatment resulted in the upregulation of SLC7A11 and GPX4 expression in HUVECs, highlighting its potent ability to mitigate ferroptosis among tested flavonoids.
Conclusions: Kaempferol effectively inhibited RSL3-induced ferroptosis in HUVECs by modulating the expression of SLC7A11 and GPX4, thereby reducing lipid peroxidation. These findings underscore the therapeutic potential of Kaempferol in the treatment of vascular diseases, paving the way for its application in clinical settings.
Introduction
The vascular endothelium, which is dynamic, diverse, and widespread, plays a crucial role in secretion, production, breakdown, and defense mechanisms1, 2 . Endothelial cells (ECs), forming the innermost layer of all blood vessels, have direct exposure to chemicals or particles within the circulatory system. They are pivotal in promoting multi-organ health and homeostasis through the regulation of solute permeability, shear stress response, vasodilatory tone maintenance, and their ability to exhibit both anti-inflammatory and pro-inflammatory, as well as antioxidant and pro-oxidant activities3, 4. Evidence increasingly supports the involvement of endothelial cell death in the onset and progression of vascular diseases5, 6, 7.
Cell death, an evolutionary conserved process, serves to regulate cell populations by eliminating excessive, damaged, or senescent cells8. Among the mechanisms of cell death, regulated cell death (RCD) stands out as crucial for maintaining tissue equilibrium and is implicated in a multitude of diseases. RCD includes both apoptotic and non-apoptotic forms9, with several non-apoptotic RCDs identified, such as necroptosis, ferroptosis, pyroptosis, and autophagy-dependent cell death10. Ferroptosis, a type of iron-dependent regulated necrosis, stems from extensive lipid peroxidation-induced membrane damage, causing iron and lipid peroxidation toxicity. This process is evolutionarily conserved and vital in both the development and pathogenesis of diverse organisms, including plants and animals11. Ferroptosis regulation involves enzymes like acyl-CoA synthetase long-chain family member 4 (ACSL4), lysophosphatidylcholine acyltransferase 3 (LPCAT3), arachidonic acid lipoxygenases (ALOXs), and glutathione peroxidase 4 (GPX4)12, highlighting the potential of targeting endothelial cell death in vascular disease treatments.
Chinese medicines and their active components offer a novel approach to modulating ferroptosis, characterized by diverse regulatory targets, structural stability, high safety profile, and affordability. Various traditional Chinese medicine ingredients have shown efficacy in disease treatment by targeting ferroptosis pathways. For example, luteolin inhibits ferroptosis in cardiac microvascular endothelial cells by enhancing interferon regulatory factor (IRF) in the context of cardiac hypertrophy13. Similarly, procyanidins (PCs) counteract oxidative stress and ferroptosis through the activation of the nuclear factor erythroid-derived 2-like 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway14. Investigating Chinese medicines' intervention mechanisms in ferroptosis opens new avenues for the research and development of innovative disease therapies15.
Flavonoids, recognized for their potent antioxidant, anti-inflammatory, anti-cancer, and anti-viral properties16, are abundantly found in fruits, vegetables, and tea. Their medicinal benefits make them integral to pharmaceuticals, dietary supplements, and beauty products17. Liu L et al. have comprehensively reviewed the regulatory functions of natural flavonoids on ferroptosis, underscoring their clinical therapy potential18. Kaempferol, abundant in plant-based foods like kale, broccoli, beans, spinach, and tea19, illustrates the therapeutic spectrum of flavonoids, including anti-oxidative16, anti-inflammatory20, and anti-cancer effects21. Its efficacy in managing conditions such as diabetes mellitus22, atherosclerosis23, and osteoporosis24 has been well-documented. Furthermore, kaempferol's neuroprotective25 and liver26 and myocardium27 benefits position it as a promising candidate for alleviating inflammatory responses28. Despite these findings, the specific impact of kaempferol on endothelial cell ferroptosis and its potential in vascular disease therapy warrants further exploration. This study aims to elucidate kaempferol's protective mechanisms against endothelial cell ferroptosis.
Methods
Cell Culture
In our study, we utilized Human Umbilical Vein Endothelial Cells (HUVECs) sourced from our research group's cell bank. The culture method we employed was based on the protocol described by Li et al.29. We maintained HUVECs in a controlled environment at 37°C within a 5% CO2 incubator, using Dulbecco’s Modified Eagle’s Medium (DMEM; C11885500BT, Gibco), enriched with 10% Fetal Bovine Serum (FBS; FSP500, ExCell) and 1% Penicillin-Streptomycin Solution (P1400, Solarbio).
Cytotoxicity Assessment
For assessing cytotoxicity, we seeded HUVECs in 96-well plates and treated them with varying concentrations of kaempferol (ranging from 0.625 to 40 µM in DMSO) for 24 hours. To evaluate cell viability, we added 10 µl of the Cell Counting Kit-8 (CCK-8; 40203ES60, YEASEN) solution to each well and incubated them for 3 hours at 37°C in a 5% CO2 environment. Subsequently, we measured the absorbance at 450 nm using a spectrophotometer.
Cell Survival Experiment
For the cell survival experiment, HUVECs were plated in 96-well plates and treated either with the GSH peroxidase 4 inhibitor RSL3 (Y-100218A, MCE) or kaempferol. After incubation at 37°C in a 5% CO2 incubator for 3 hours, we measured absorbance at 450 nm using a spectrophotometer for cell viability assessment.
Detection of Reactive Oxygen Species (ROS)
To detect ROS production, we utilized the Superoxide Anion Probe Dihydroethidium (DHE) assay30. This involved culturing HUVECs and subsequently incubating them with 10 μM DHE (S0063, Beyotime) at 37°C for 30 minutes. After washing the cells twice with phosphate-buffered saline (PBS) and fixing them with 4% paraformaldehyde for 30 minutes, we applied an anti-fluorescence quenching agent containing 4',6-diamidino-2-phenylindole (DAPI) (ZLI-9556, ZSGB-BIO) for counter-staining. We then examined and photographed the cells under a confocal microscope.
Lipid Peroxidation Measurement
Following the methodology of Mei et al.31, we detected lipid peroxidation using the C11 BODIPY 581/591 indicator. After pretreating HUVECs, we added C11 BODIPY 581/591 (D3861, Invitrogen) at a final concentration of 5 μM to the culture medium and co-incubated it for one hour at 37°C. We washed the cells twice with PBS, treated them with trypsin, resuspended them in PBS containing 5% FBS, and finally analyzed them using flow cytometry.
Western Blot Analysis
We analyzed the intracellular protein content using the Western Blot technique32. After pretreatment, HUVECs were lysed with RIPA buffer containing protease inhibitors on ice for 30 minutes. The proteins were then separated by SDS-PAGE and transferred onto PVDF membranes. After blocking with 5% skim milk, the membranes were incubated with primary antibodies against SLC7A11 (ab175186, Abcam), GPX4 (A1933, ABclonal), and GAPDH (A19056, ABclonal) overnight at 4°C, using a 1:1000 dilution. The next day, we incubated the membranes with an HRP-conjugated Goat Anti-Rabbit secondary antibody (RGAR001, Proteintech) at a 1:10,000 dilution for one hour at room temperature. Detection was achieved using a chemiluminescent substrate. GAPDH served as a loading control33, 34, and the bands were quantitatively analyzed using ImageJ software (version 1.4.3.67).
Realtime Fluorescence Quantitative PCR (qPCR)
We extracted total RNA using TRI Reagent (T9424, Sigma) and synthesized cDNA with TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix (AT311-02, Transgen). We performed qPCR using the QuantStudioTM 3 System and PerfectStart® Green qPCR SuperMix (+Universal Passive Reference Dye) (AQ602-01, Transgen), detecting fluorescence with SYBR Green. The amplification efficiency was calculated as E = 10(-1/k)-1, with efficiencies for SLC7A11 and GPX4 at 99.8% and 102.7%, respectively. We normalized the expression levels of SLC7A11 and GPX4 mRNAs to β-actin mRNA using the 2-ΔΔCt method and employed the following primers for qPCR:
- β-actin: - Forward: 5′-CCTGGCACCCAGCACAAT-3′ - Reverse: 5′-GGGCCGGACTCGTCATAC-3′- SLC7A11: - Forward: 5′-ATGCAGTGGCAGTGACCTTT-3′ - Reverse: 5′-CATGGAGCCAAAGCAGGAGA-3′- GPX4: - Forward: 5′-GAAGATCCAACCCAAGGGCA-3′ - Reverse: 5′-GACGGTGTCCAAACTTGGTG-3′
Statistical Analysis
We meticulously analyzed all data to ensure a normal distribution and presented the results as the mean ± standard deviation (SD). Statistical significance was determined using Student's t-test or one-way ANOVA, followed by post-hoc testing. We utilized Pearson's product-moment correlation for correlation analyses. All statistical procedures were conducted using GraphPad Prism version 8.0.2, considering p-values of < 0.05 as statistically significant.
Results
Kaempferol Protects HUVECs from RSL-3-Induced Ferroptosis
Ferroptosis was induced in HUVECs using different concentrations of RSL-3 (DMSO, 0.125, 0.25, 0.5, and 1 μM). A dose-dependent decrease in cell viability in HUVECs was detected. The LD50 of RSL-3 in cell viability was achieved at a concentration of 0.25 μM. Subsequent induction experiments were carried out using this concentration (Figure 1A). To screen for the most active flavonoids, HUVECs were treated with different flavonoid components and co-cultured with RSL-3 for 24 hours. Kaempferol demonstrated the strongest ability to rescue the RSL-3-induced ferroptosis (Figure 1B). Figure 1C shows the chemical structural formula of Kaempferol.
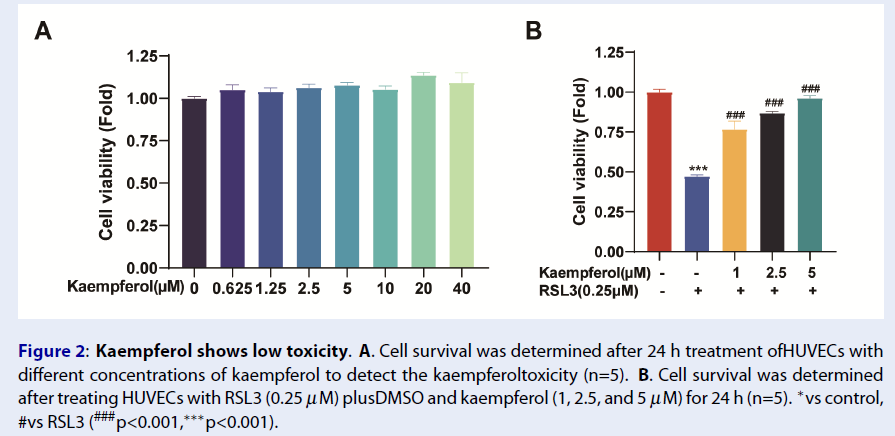
Kaempferol Shows Low Toxicity in HUVEC Culture
The toxicity of kaempferol on HUVECs was analyzed using the CCK-8 assay. HUVECs were treated with different concentrations of kaempferol (DMSO, 0.625, 1.25, 2.5, 5, 10, 20, and 40 μM) for 24 hours, and the cell viability was analyzed. Interestingly, kaempferol showed no toxicity, but a slight growth-promoting effect (Figure 2A). Moreover, kaempferol was shown to resist ferroptosis and effectively rescue HUVECs similar to the control group at a concentration of 5 μM. The subsequent experiments were performed with this concentration to rescue HUVEC ferroptosis (Figure 2B).
Kaempferol Reduces ROS Generation in RSL-3-Treated HUVECs
Ferroptosis is a ROS-dependent, non-apoptotic, lipid-peroxidation-induced cell death closely related to the intracellular ROS content35, 36. ROS are generated during normal physiological processes and are essential for cell signaling and tissue homeostasis37. The C11 BODIPY 581/591 and DHE assays were used to determine the lipid oxidation profiles and oxidative stress in HUVECs, respectively. The results showed that the fluorescence intensity of DHE increased significantly following RSL-3 treatment and C11 BODIPY 581/591 addition, whereas both DHE and C11 BODIPY 581/591 decreased significantly upon treatment with kaempferol, indicating the antioxidant capacity of kaempferol (Figure 3).
Kaempferol Inhibits Ferroptosis by Upregulating SLC7A11 and GPX4
The protein expression of SLC7A11 and GPX4 was significantly downregulated in the RSL-3 group. However, treatment with kaempferol restored the protein levels to that of the control, suggesting that kaempferol inhibits ferroptosis in HUVECs (Figure 4 A, B, C). This correlation was also verified at the RNA level, with qPCR results being consistent with the western blot findings (Figure 4 D, E).
Discussion
The pathology of vascular diseases often involves the dysregulation of endothelial cell death, making the study of endothelial cell death models crucial for the identification of effective treatments for such diseases. Human Umbilical Vein Endothelial Cells (HUVECs) are frequently utilized in research on cell biology within the contexts of angiogenesis, vascular diseases, and cardiovascular diseases38. Due to their superior proliferation and migration abilities, as well as their capacity to form in vitro tubular structures resembling angiogenesis, HUVECs are considered ideal models for exploring endothelial cell death38.
Ferroptosis, a form of non-apoptotic cell death characterized by iron-dependent lipid peroxidation, is distinguished by an accumulation of lipid peroxides leading to cell swelling and the subsequent rupture of the cell membrane8, 39. The process of lipid peroxidation is fundamental to ferroptosis40. Compounds such as RSL3 and erastin, known inducers of ferroptosis, are employed to develop cell models for this form of cell death. Erastin targets System Xc- activity to disrupt glutathione (GSH) synthesis, a pathway leading to ferroptosis41, whereas RSL3, by directly inhibiting GPX4, activates iron-dependent, nonapoptotic cell death in cells with RAS mutations42, 43 and in various cell types44, 45. Observations of morphological changes, including cell and mitochondrial shrinkage as well as cell membrane damage, in HUVECs treated with RSL3 confirm its efficacy in creating an ideal model for endothelial cell ferroptosis.
Recent decades have seen the identification of pharmacological and natural compounds capable of modulating ferroptosis46, 47. Among these, kaempferol, a compound found in abundance in fruits, vegetables, and herbs, stands out for its minimal toxicity and promising therapeutic potential48. Its mechanisms involve the promotion of free radical scavenging, enhancement of antioxidant enzyme activities against lipid peroxidation, and prevention of hemolysis49. Moreover, kaempferol acts protectively in ischemic stroke by activating specific signaling pathways (Nrf2/SLC7A11/GPX4) to mitigate oxygen-glucose deprivation/reperfusion-induced cellular damage and suppress ferroptosis initiation50. Additionally, it reverses adverse effects such as hepatic iron overload and oxidative stress induced by acetaminophen in mice, showcasing its ability to reduce intracellular ROS accumulation, trigger the Nrf2 pathway, upregulate GPX4, and prevent hepatocyte ferroptosis51. Through our research, using HUVECs as a model, we established kaempferol's efficacy in attenuating RSL3-induced cell death, highlighting its potential in the treatment of vascular diseases through ferroptosis inhibition.
In exploring ferroptosis further, we discovered it to be a reactive oxygen species (ROS)-dependent cell demise mechanism, exacerbating oxidative damage through excessive ROS generation via the Fenton reaction52, 53. Our investigation into the impact of kaempferol on lipid peroxidation, utilizing assays like DHE for intracellular ROS levels30 and C11-BODIPY for lipid peroxidation40, confirmed significant inhibition of RSL3-induced lipid peroxidation in HUVECs.
Multiple regulatory signals such as GPX4 and SLC7A11 are involved in the regulation of cell ferroptosis54. The GSH–GPX4 limits membrane lipid peroxidation via targeting System Xc¯ cystine/glutamate antiporter55, 56. SLC7A11 maintains the production of GSH, a major endogenous antioxidant, through a series of reactions involving the exchange of extracellular cysteine with intracellular glutamate46. Inhibiting the SLC7A11 pathway stands out as a critical upstream mechanism for inducing ferroptosis57. The expression of GPX4 and SLC7A11 at both protein and RNA levels was investigated in RSL3-treated HUEVCs. This study also provides evidence that kaempferol could significantly protect HUEVCs ferroptosis through the regulation of GPX4 and SLC7A11 expression.
The implications of endothelial cell ferroptosis extend to a variety of vascular-related conditions, including peripheral vascular disease58, stroke59, heart disease60, diabetes61, venous thrombosis62, tumor growth63, and metastasis64, making the targeting of endothelial cell ferroptosis a novel therapeutic strategy. Kaempferol's multi-faceted pharmacological effects, combined with its minimal toxicity, endow it with significant potential in both health food and pharmaceutical sectors51.
In previous studies, kaempferol has shown potential effectiveness in the treatment of diseases such as Alzheimer's disease65 and colon cancer. It exhibits various effects such as antioxidant, anti-inflammatory, anti-tumor, and promotion of glucose metabolism by regulating multiple signaling pathways such as Nrf2/SLC7A11/GPX4, Toll-like receptor 4 (TLR4)/ nuclear factor kappa-B (NF-κB), immunoglobulin-regulated enhancer 1 (IRE1)/ c-Jun N-terminal kinase (JNK)/ C/EBP homology protein (CHOP), and mitogen-activated protein kinases (MAPKs). In addition, compared with some chemotherapeutic agents, kaempferol is not toxic to normal cells66 and appears to be relatively safe at certain doses67. However, clinical trials of kaempferol on humans are still scarce and remain controversial, as most studies are based on animal models or in vitro experiments. More studies are still needed to determine its safety, pharmacokinetics and potential adverse effects in humans. In addition, although kaempferol has shown potential therapeutic effects in vitro and in vivo models, there is a lack of clinical trial validation, and therefore more human studies are needed to confirm its efficacy and safety in clinical applications.
The present study had a limited experimental model and did not elucidate the molecular mechanisms of kaempferol in depth. Future studies could further explore the molecular mechanisms and interactions of kaempferol in regulating signaling pathways and inhibiting iron death, as well as the targets and biological effects of kaempferol. This will contribute to a better understanding of the mechanism of action of kaempferol and provide a more scientific basis for its future clinical applications.
Conclusions
We uncovered the protective role of kaempferol in safeguarding human umbilical vein endothelial cells (HUVECs) from ferroptosis, an iron-dependent form of cell death. This protective mechanism functions through the modulation of GPX4 and SLC7A11, crucial elements in the cell's defense against ferroptosis. These insights broaden our comprehension of ferroptosis mechanisms and position kaempferol as a potential therapeutic candidate for drug development.
Abbreviations
ALOXs - Arachidonic Acid Lipoxygenases, ANOVA - Analysis of Variance, ACSL4 - Acyl-CoA Synthetase Long-Chain Family Member 4, CCK-8 - Cell Counting Kit-8, DAPI - 4',6-diamidino-2-phenylindole, DMEM - Dulbecco’s Modified Eagle’s Medium, DHE - Dihydroethidium, FBS - Fetal Bovine Serum, GSH - Glutathione, GPX4 - Glutathione Peroxidase 4, HO-1 - Heme Oxygenase-1, HRP - Horseradish Peroxidase, HUVECs - Human Umbilical Vein Endothelial Cells, IRF - Interferon Regulatory Factor, LPCAT3 - Lysophosphatidylcholine Acyltransferase 3, Nrf2 - Nuclear Factor Erythroid-Derived 2-Like 2, PBS - Phosphate-Buffered Saline, PCs - Procyanidins, PVDF - Polyvinylidene Difluoride, qPCR - Real-Time Fluorescence Quantitative Polymerase Chain Reaction, RCD - Regulated Cell Death, RIPA - Radioimmunoprecipitation Assay Buffer, ROS - Reactive Oxygen Species, SD - Standard Deviation, SDS-PAGE - Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis, SLC7A11 - Solute Carrier Family 7 Member 11
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Author’s contributions
All authors significantly contributed to this work, read and approved the final manuscript.
Funding
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Zhang
H.,
Vreeken
D.,
Bruikman
C.S.,
van Zonneveld
A.J.,
van Gils
J.M.,
Understanding netrins and semaphorins in mature endothelial cell biology. Pharmacological Research.
2018;
137
:
1-10
.
View Article PubMed Google Scholar -
Liu
Y.V.,
Santiago
C.P.,
Sogunro
A.,
Konar
G.J.,
Hu
M.W.,
McNally
M.M.,
Single-cell transcriptome analysis of xenotransplanted human retinal organoids defines two migratory cell populations of nonretinal origin. Stem Cell Reports.
2023;
18
(5)
:
1138-54
.
View Article PubMed Google Scholar -
Salewskij
K.,
Penninger
J.M.,
Blood Vessel Organoids for Development and Disease. Circulation Research.
2023;
132
(4)
:
498-510
.
View Article PubMed Google Scholar -
Zheng
D.,
Liu
J.,
Piao
H.,
Zhu
Z.,
Wei
R.,
Liu
K.,
ROS-triggered endothelial cell death mechanisms: focus on pyroptosis, parthanatos, and ferroptosis. Frontiers in Immunology.
2022;
13
.
View Article PubMed Google Scholar -
Kulovic-Sissawo
A.,
Tocantins
C.,
Diniz
M.S.,
Weiss
E.,
Steiner
A.,
Tokic
S.,
Mitochondrial Dysfunction in Endothelial Progenitor Cells: Unraveling Insights from Vascular Endothelial Cells. Biology (Basel).
2024;
13
(2)
:
70
.
View Article PubMed Google Scholar -
Liu
Z.,
Zhang
X.,
Xiong
S.,
Huang
S.,
Ding
X.,
Xu
M.,
Endothelial dysfunction of syphilis: Pathogenesis. Journal of the European Academy of Dermatology and Venereology.
2024;
2024
:
Early view
.
View Article Google Scholar -
Mozzicato
A.M.,
Bastrup
J.A.,
Sanchez-Alonso
J.L.,
van der Horst
J.,
Gorelik
J.,
Hägglund
P.,
Mesenteric artery smooth muscle cells from hypertensive rats have increased microtubule acetylation. The Biochemical Journal.
2024;
481
(5)
:
387-403
.
View Article PubMed Google Scholar -
Chen
X.,
Kang
R.,
Kroemer
G.,
Tang
D.,
Ferroptosis in infection, inflammation, and immunity. The Journal of Experimental Medicine.
2021;
218
(6)
.
View Article PubMed Google Scholar -
Tang
D.,
Kang
R.,
Berghe
T.V.,
Vandenabeele
P.,
Kroemer
G.,
The molecular machinery of regulated cell death. Cell Research.
2019;
29
(5)
:
347-64
.
View Article PubMed Google Scholar -
Galluzzi
L.,
Vitale
I.,
Aaronson
S.A.,
Abrams
J.M.,
Adam
D.,
Agostinis
P.,
Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death and Differentiation.
2018;
25
(3)
:
486-541
.
View Article PubMed Google Scholar -
Distéfano
A.M.,
Martin
M.V.,
Córdoba
J.P.,
Bellido
A.M.,
D'Ippólito
S.,
Colman
S.L.,
Heat stress induces ferroptosis-like cell death in plants. The Journal of Cell Biology.
2017;
216
(2)
:
463-76
.
View Article PubMed Google Scholar -
Liu
J.,
Kang
R.,
Tang
D.,
Signaling pathways and defense mechanisms of ferroptosis. The FEBS Journal.
2022;
289
(22)
:
7038-50
.
View Article PubMed Google Scholar -
Liu
Y.,
Yang
G.,
Huo
S.,
Wu
J.,
Ren
P.,
Cao
Y.,
Lutein suppresses ferroptosis of cardiac microvascular endothelial cells via positive regulation of IRF in cardiac hypertrophy. European Journal of Pharmacology.
2023;
959
.
View Article PubMed Google Scholar -
Chen
L.,
Huang
J.,
Yao
Z.M.,
Sun
X.R.,
Tong
X.H.,
Hu
M.,
Procyanidins Alleviated Cerebral Ischemia/Reperfusion Injury by Inhibiting Ferroptosis via the Nrf2/HO-1 Signaling Pathway. Molecules (Basel, Switzerland).
2023;
28
(8)
:
3582
.
View Article PubMed Google Scholar -
Xu
W.H.,
Li
C.H.,
Jiang
T.L.,
[Ferroptosis pathway and its intervention regulated by Chinese materia medica]. Zhongguo Zhongyao Zazhi.
2018;
43
(20)
:
4019-26
.
PubMed Google Scholar -
S.
Chagas, M. D. S.,
D.
Behrens, M.,
J.
Moragas-Tellis, C.,
X.
Penedo, G.,
R.
Silva, A.,
F.
Gonçalves-de-Albuquerque, C.,
Flavonols and Flavones as Potential anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxidative Medicine and Cellular Longevity.
2022;
2022
:
9966750
.
View Article Google Scholar -
Muruganathan
N.,
Dhanapal
A.R.,
Baskar
V.,
Muthuramalingam
P.,
Selvaraj
D.,
Aara
H.,
Recent Updates on Source, Biosynthesis, and Therapeutic Potential of Natural Flavonoid Luteolin: A Review. Metabolites.
2022;
12
(11)
:
1145
.
View Article PubMed Google Scholar -
Liu
L.,
Wang
L.,
Xiao
Y.,
Liu
Y.,
Meng
X.,
Shen
X.,
Natural flavonoids act as potent ferroptosis inhibitors and their potentials in the treatment of ferroptosis-associated diseases. Pharmacological Research. Modern Chinese Medicine.
2024;
10
.
View Article Google Scholar -
Periferakis
A.,
Periferakis
K.,
Badarau
I.A.,
Petran
E.M.,
Popa
D.C.,
Caruntu
A.,
Kaempferol: antimicrobial properties, sources, clinical, and traditional applications. International Journal of Molecular Sciences.
2022;
23
(23)
:
15054
.
View Article Google Scholar -
Alam
W.,
Khan
H.,
Shah
M.A.,
Cauli
O.,
Saso
L.,
Kaempferol as a Dietary Anti-Inflammatory Agent: Current Therapeutic Standing. Molecules (Basel, Switzerland).
2020;
25
(18)
:
4073
.
View Article PubMed Google Scholar -
Akter
M.,
Parvin
M.S.,
Hasan
M.M.,
Rahman
M.A.,
Islam
M.E.,
Anti-tumor and antioxidant activity of kaempferol-3-O-alpha-L-rhamnoside (Afzelin) isolated from Pithecellobium dulce leaves. BMC Complementary Medicine and Therapies.
2022;
22
(1)
:
169
.
View Article PubMed Google Scholar -
Yang
Y.,
Chen
Z.,
Zhao
X.,
Xie
H.,
Du
L.,
Gao
H.,
Mechanisms of Kaempferol in the treatment of diabetes: A comprehensive and latest review. Frontiers in endocrinology.
2022;
13
:
990299
.
View Article Google Scholar -
Chen
M.,
Xiao
J.,
El-Seedi
H.R.,
Woźniak
K.S.,
Daglia
M.,
Little
P.J.,
Kaempferol and atherosclerosis: From mechanism to medicine. Critical Reviews in Food Science and Nutrition.
2024;
64
(8)
:
2157-2175
.
View Article Google Scholar -
Liu
H.,
Yi
X.,
Tu
S.,
Cheng
C.,
Luo
J.,
Kaempferol promotes BMSC osteogenic differentiation and improves osteoporosis by downregulating miR-10a-3p and upregulating CXCL12. Molecular and Cellular Endocrinology.
2021;
520
.
View Article PubMed Google Scholar -
Chang
S.,
Li
X.,
Zheng
Y.,
Shi
H.,
Zhang
D.,
Jing
B.,
Kaempferol exerts a neuroprotective effect to reduce neuropathic pain through TLR4/NF‐ĸB signaling pathway. Phytotherapy Research.
2022;
36
(4)
:
1678-91
.
View Article PubMed Google Scholar -
Xiao
X.,
Hu
Q.,
Deng
X.,
Shi
K.,
Zhang
W.,
Jiang
Y.,
Old wine in new bottles: kaempferol is a promising agent for treating the trilogy of liver diseases. Pharmacological Research.
2022;
175
.
View Article PubMed Google Scholar -
Kamisah
Y.,
Jalil
J.,
Yunos
N.M.,
Zainalabidin
S.,
Cardioprotective Properties of Kaempferol: A Review. Plants.
2023;
12
(11)
:
2096
.
View Article PubMed Google Scholar -
Cheng
X.,
Yang
Y.L.,
Yang
H.,
Wang
Y.H.,
Du
G.H.,
Kaempferol alleviates LPS-induced neuroinflammation and BBB dysfunction in mice via inhibiting HMGB1 release and down-regulating TLR4/MyD88 pathway. International Immunopharmacology.
2018;
56
:
29-35
.
View Article PubMed Google Scholar -
Li
M.,
Zhang
D.,
Zhang
L.,
Wang
L.S.,
Tian
Y.,
Li
B.,
The inhibitory effect of chemical components from Ginkgo biloba flower on ferroptosis in vascular endothelial cells. Journal of International Pharmaceutical Research..
2020;
47
:
857-62
.
-
Gardiner
B.,
Dougherty
J.A.,
Ponnalagu
D.,
Singh
H.,
Angelos
M.,
Chen
C.A.,
Measurement of Oxidative Stress Markers In Vitro Using Commercially Available Kits. 2020
.
View Article Google Scholar -
Mei
H.,
Zhao
L.,
Li
W.,
Zheng
Z.,
Tang
D.,
Lu
X.,
Inhibition of ferroptosis protects House Ear Institute-Organ of Corti 1 cells and cochlear hair cells from cisplatin-induced ototoxicity. Journal of Cellular and Molecular Medicine.
2020;
24
(20)
:
12065-81
.
View Article PubMed Google Scholar -
Zhou
D.,
Liang
Q.,
Ge
X.,
Xu
J.,
Allogeneic platelet-rich plasma inhibits ferroptosis in promoting wound repair of type 2 diabetic ulcers. Free Radical Biology & Medicine.
2024;
215
:
37-47
.
View Article PubMed Google Scholar -
Matsuda
N.,
Imbaby
S.,
Hattori
K.,
Hattori
Y.,
STAT3 decoy oligodeoxynucleotides improve end-organ tissue injury and survival in septic mice. The FASEB Journal.
2020;
34
:
1-1
.
View Article Google Scholar -
Imbaby
S.,
Hattori
Y.,
Stattic ameliorates the cecal ligation and puncture-induced cardiac injury in septic mice via IL-6-gp130-STAT3 signaling pathway. Life Sciences.
2023;
330
.
View Article PubMed Google Scholar -
Su
L.J.,
Zhang
J.H.,
Gomez
H.,
Murugan
R.,
Hong
X.,
Xu
D.,
Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Medicine and Cellular Longevity.
2019;
2019
.
View Article PubMed Google Scholar -
Kapralov
A.A.,
Yang
Q.,
Dar
H.H.,
Tyurina
Y.Y.,
Anthonymuthu
T.S.,
Kim
R.,
Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nature Chemical Biology.
2020;
16
(3)
:
278-90
.
View Article PubMed Google Scholar -
Ferreira
C.A.,
Ni
D.,
Rosenkrans
Z.T.,
Cai
W.,
Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Research.
2018;
11
(10)
:
4955-84
.
View Article PubMed Google Scholar -
Baselet
B.,
Sonveaux
P.,
Baatout
S.,
Aerts
A.,
Pathological effects of ionizing radiation: endothelial activation and dysfunction. Cellular and Molecular Life Sciences.
2019;
76
(4)
:
699-728
.
View Article PubMed Google Scholar -
Stockwell
B.R.,
Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell.
2022;
185
(14)
:
2401-21
.
View Article PubMed Google Scholar -
Dai
Z.,
Zhang
W.,
Zhou
L.,
Huang
J.,
Probing lipid peroxidation in ferroptosis: emphasizing the utilization of C11-BODIPY-based protocols. In: Ferroptosis: Methods and Protocols. New York, NY: Springer US, 2023. p. 61-72.
.
-
Dixon
S.J.,
Patel
D.N.,
Welsch
M.,
Skouta
R.,
Lee
E.D.,
Hayano
M.,
Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife.
2014;
3
.
View Article PubMed Google Scholar -
Campos
J.,
Gleitze
S.,
Hidalgo
C.,
Núñez
M.T.,
IP3R-Mediated Calcium Release Promotes Ferroptotic Death in SH-SY5Y Neuroblastoma Cells. Antioxidants.
2024;
13
(2)
:
196
.
View Article PubMed Google Scholar -
Schwantes
A.,
Wickert
A.,
Becker
S.,
Baer
P.C.,
Weigert
A.,
Brüne
B.,
Tumor associated macrophages transfer ceruloplasmin mRNA to fibrosarcoma cells and protect them from ferroptosis. Redox Biology.
2024;
71
.
View Article PubMed Google Scholar -
Costa
I.,
Barbosa
D.J.,
Benfeito
S.,
Silva
V.,
Chavarria
D.,
Borges
F.,
Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacology & Therapeutics.
2023;
244
.
View Article PubMed Google Scholar -
Wang
L.,
Chen
X.,
Yan
C.,
Ferroptosis: an emerging therapeutic opportunity for cancer. Genes & Diseases.
2020;
9
(2)
:
334-46
.
View Article PubMed Google Scholar -
Tang
D.,
Chen
X.,
Kang
R.,
Kroemer
G.,
Ferroptosis: molecular mechanisms and health implications. Cell Research.
2021;
31
(2)
:
107-25
.
View Article PubMed Google Scholar -
Li
J.,
Cao
F.,
Yin
H.L.,
Huang
Z.J.,
Lin
Z.T.,
Mao
N.,
Ferroptosis: past, present and future. Cell Death & Disease.
2020;
11
(2)
:
88
.
View Article PubMed Google Scholar -
Ma
X.,
Zhang
X.,
Wang
X.,
Wang
C.,
Ma
Y.,
The role of kaempferol in gynaecological malignancies: progress and perspectives. Frontiers in Pharmacology.
2023;
14
.
View Article PubMed Google Scholar -
Wu
P.,
Meng
X.,
Zheng
H.,
Zeng
Q.,
Chen
T.,
Wang
W.,
Kaempferol Attenuates ROS-Induced Hemolysis and the Molecular Mechanism of Its Induction of Apoptosis on Bladder Cancer. Molecules (Basel, Switzerland).
2018;
23
(10)
:
2592
.
View Article PubMed Google Scholar -
Yuan
Y.,
Zhai
Y.,
Chen
J.,
Xu
X.,
Wang
H.,
Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules.
2021;
11
(7)
:
923
.
View Article PubMed Google Scholar -
Li
H.,
Weng
Q.,
Gong
S.,
Zhang
W.,
Wang
J.,
Huang
Y.,
Kaempferol prevents acetaminophen-induced liver injury by suppressing hepatocyte ferroptosis via Nrf2 pathway activation. Food & Function.
2023;
14
(4)
:
1884-96
.
View Article PubMed Google Scholar -
Dong
X.,
Zhou
S.,
Nao
J.,
Kaempferol as a therapeutic agent in Alzheimer's disease: evidence from preclinical studies. Ageing Research Reviews.
2023;
87
.
View Article PubMed Google Scholar -
Wang
Y.,
Wu
J.,
Ferroptosis: a new strategy for cardiovascular disease. Frontiers in Cardiovascular Medicine.
2023;
10
.
View Article PubMed Google Scholar -
Lou
X.,
Zhang
Y.,
Guo
J.,
Gao
L.,
Ding
Y.,
Zhuo
X.,
What is the impact of ferroptosis on diabetic cardiomyopathy: a systematic review. Heart Failure Reviews.
2023;
29
(1)
:
1-11
.
View Article PubMed Google Scholar -
Xu
X.,
Xu
X.D.,
Ma
M.Q.,
Liang
Y.,
Cai
Y.B.,
Zhu
Z.X.,
The mechanisms of ferroptosis and its role in atherosclerosis. Biomedicine and Pharmacotherapy.
2024;
171
.
View Article PubMed Google Scholar -
Koeberle
S.C.,
Kipp
A.P.,
Stuppner
H.,
Koeberle
A.,
Ferroptosis-modulating small molecules for targeting drug-resistant cancer: challenges and opportunities in manipulating redox signaling. Medicinal Research Reviews.
2023;
43
(3)
:
614-82
.
View Article PubMed Google Scholar -
Fu
P.,
Chen
Y.,
Wu
M.,
Bao
B.,
Yin
X.,
Chen
Z.,
Effect of ferroptosis on chronic cerebral hypoperfusion in vascular dementia. Experimental Neurology.
2023;
370
.
View Article PubMed Google Scholar -
Jiang
X.,
Stockwell
B.R.,
Conrad
M.,
Ferroptosis: mechanisms, biology and role in disease. Nature Reviews. Molecular Cell Biology.
2021;
22
(4)
:
266-82
.
View Article PubMed Google Scholar -
Cartland
S.P.,
Stanley
C.P.,
Bursill
C.,
Passam
F.,
Figtree
G.A.,
Patel
S.,
Sex, Endothelial Cell Functions, and Peripheral Artery Disease. International Journal of Molecular Sciences.
2023;
24
(24)
:
17439
.
View Article PubMed Google Scholar -
Jung
J.M.,
Gruber
A.,
Heseltine
P.,
Rajamani
K.,
Ameriso
S.F.,
Fisher
M.J.,
New Directions in Infection-Associated Ischemic Stroke. Journal of Clinical Neurology (Seoul, Korea).
2024;
20
(2)
:
140-52
.
View Article PubMed Google Scholar -
Yubero-Serrano
E.M.,
Fernandez-Gandara
C.,
Garcia-Rios
A.,
Rangel-Zuñiga
O.A.,
Gutierrez-Mariscal
F.M.,
Torres-Peña
J.D.,
Mediterranean diet and endothelial function in patients with coronary heart disease: an analysis of the CORDIOPREV randomized controlled trial. PLoS Medicine.
2020;
17
(9)
.
View Article PubMed Google Scholar -
Yin
J.,
Fu
X.,
Luo
Y.,
Leng
Y.,
Ao
L.,
Xie
C.,
Narrative Review of Diabetic Macroangiopathy: From Molecular Mechanism to Therapeutic Approaches. Diabetes Therapy.
2024;
15
:
585-609
.
View Article Google Scholar -
Bartimoccia
S.,
Praktiknjo
M.,
Nocella
C.,
Schierwagen
R.,
Cammisotto
V.,
Jansen
C.,
Association between endotoxemia and blood no in the portal circulation of cirrhotic patients: results of a pilot study. Internal and Emergency Medicine.
2024;
19
(3)
:
713-20
.
View Article PubMed Google Scholar -
Díaz-Flores
L.,
Gutiérrez
R.,
González-Gómez
M.,
García
M.D.,
Carrasco-Juan
J.L.,
Martín-Vasallo
P.,
Phenomena of Intussusceptive Angiogenesis and Intussusceptive Lymphangiogenesis in Blood and Lymphatic Vessel Tumors. Biomedicines.
2024;
12
(2)
:
258
.
View Article PubMed Google Scholar -
Zhou
J.,
Zhou
P.,
Wang
J.,
Song
J.,
Roles of endothelial cell specific molecule 1 in tumor angiogenesis (Review). Oncology Letters.
2024;
27
(3)
:
137
.
View Article PubMed Google Scholar -
Luo
H.,
Jiang
B.H.,
King
S.M.,
Chen
Y.C.,
Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutrition and Cancer.
2008;
60
(6)
:
800-9
.
View Article PubMed Google Scholar -
Bangar
S.P.,
Chaudhary
V.,
Sharma
N.,
Bansal
V.,
Ozogul
F.,
Lorenzo
J.M.,
Kaempferol: A flavonoid with wider biological activities and its applications. Critical Reviews in Food Science and Nutrition.
2023;
63
(28)
:
9580-604
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 4 (2024)
Page No.: 6339-6347
Published on: 2024-04-30
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 2384 times
- PDF downloaded - 725 times
- XML downloaded - 49 times
 Biomedpress
Biomedpress





