Abstract
Osteomyelitis, a serious condition marked by inflammation of the medullary cavity, Haversian system, and adjacent bone cortex, requires urgent medical attention, especially when it involves the jaw. This condition is particularly concerning due to its potential to cause significant facial disfigurement and the increased risk of the infection spreading to the skull base or fascial spaces of the head and neck. Among the facial bones, the mandible is more commonly affected by osteomyelitis than the maxilla. This discrepancy is attributed to the anatomical and physiological differences between these bones; the maxilla benefits from a rich collateral blood flow and possesses a thin cortical bone, both of which reduce its vulnerability to infections. In this case series, we present three cases of osteomyelitis in patients who had underlying debilitating conditions such as diabetes mellitus and deleterious habits, which acted as predisposing factors for the development of the disease. These cases highlight the complex interplay between systemic health issues and the susceptibility to localized bone infections. The first two cases involve a patient with poorly controlled diabetes, emphasizing how chronic hyperglycemia can impair immune function and compromise healing, thereby facilitating the onset and progression of osteomyelitis. The third case features a patient with a history of tobacco use, illustrating the detrimental impact of deleterious habits on vascular health and immune response. In addition to detailing these cases, this series provides an overview of the current understanding of the pathogenesis and treatment strategies for osteomyelitis. Treatment typically involves a combination of surgical debridement, antimicrobial therapy, and management of underlying conditions to enhance immune function and promote recovery.
Introduction
The term "Osteomyelitis" was coined in 1852 by French surgeon Edouard Chassaignac1. It derives from three Greek words: "osteon" (bone), "myelon" (marrow), and "itis" (inflammation), collectively describing an inflammatory condition affecting the bone marrow. This disease typically starts as an infection within the medullary cavity, rapidly affecting the haversian system and extending to the periosteum2. The prevalence and incidence of osteomyelitis in the jawbones vary globally due to factors such as geographic location, population demographics, the broad spectrum of microbiological agents involved, and the systemic health of individuals. Jawbone osteomyelitis demands significant clinical attention due to its potential for severe complications, including sepsis, cellulitis, life-threatening conditions, pathological fractures, orocutaneous fistulas, functional impairments, septic shock, and organ failure3. Early recognition of its signs, symptoms, and effective treatment strategies is essential for both dental and medical professionals, as the condition often correlates with systemic diseases like diabetes and detrimental habits such as smoking and alcohol consumption. This necessitates a multidisciplinary approach for managing osteomyelitis cases effectively. A thorough understanding of osteomyelitis is crucial for timely diagnosis, appropriate intervention, and coordinated management, leading to enhanced patient outcomes. This case series aims to highlight the importance of recognizing the diverse etiological factors contributing to osteomyelitis and the therapeutic challenges encountered today. It endeavors to illuminate the complexity of this condition and the necessity for personalized diagnostic and therapeutic strategies in clinical settings.
Case Report 1
A 55-year-old female patient presented with a complaint of a non-healing extraction socket in the upper left back tooth region, persisting for six months. The patient had undergone an atraumatic dental extraction six months prior. Two months post-extraction, she developed pain and pus discharge at the site and sought treatment from a private dentist. The treatment included debridement and a 5-day course of oral antibiotics, following which the symptoms initially subsided. However, there was a sudden exacerbation marked by pus discharge three weeks later.
Clinical examination revealed a single, well-defined bony defect in the left maxillary labial alveolus in the region of teeth 23 and 24, encased by a thin layer of necrotic bone. The center of this defect was covered with a yellowish-white pseudomembranous slough, measuring approximately 1x1cm. Palpatory examination showed the margins of the bony defect to be sharp and non-tender, with mild pus discharge present (Figure 1A).
Based on these findings, a provisional diagnosis of osteomyelitis of the left maxillary alveolus was established, with mucormycosis as a differential diagnosis. The patient underwent antibiotic sensitivity testing, revealing a mild strain of Klebsiella pneumoniae, which showed high sensitivity to ciprofloxacin, gentamycin, clotrimazole, and cefotaxime. Biochemical investigations indicated HbA1c at 7.3%, fasting blood glucose of 145 mg/dL, and postprandial blood glucose of 293 mg/dL.
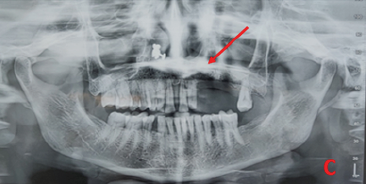
Further radiographic investigations included an intraoral periapical radiograph (IOPA), which demonstrated alterations in the upper margin of the alveolar ridge and erosive changes in the regions of teeth 23 and 24 (Figure 1B). An orthopantomogram (OPG) showed erosion of the alveolar bone in relation to tooth 23 extending to the hard palate, with evidence of an unhealed socket in teeth 24 and 25 (Figure 1C). A computed tomography (CT) scan revealed evidence of permeative bony erosion with thinning and rarefaction of the alveolar bone of the left maxilla, disruption in the continuity of the labial and palatal cortex, and minimal protrusion of soft tissue content into the left maxillary sinus, suggestive of osteomyelitis of the left maxilla (Figure 1D).
The patient then underwent curettage of the necrotic bone under local anesthesia, and the excised specimen was sent for histopathological examination. The analysis revealed numerous spicules of sclerotic and necrotic bone marrow, with a few bacterial colonies. The bone trabeculae exhibited irregular areas of resorption and empty lacunae, alongside dense lymphoplasmacytic infiltration and areas of fibrosis, indicative of sclerosing osteomyelitis.
Case Report 2
A 53-year-old female patient presented with complaints of a non-healing ulcer in the region posterior to the maxillary left canine, persisting for the past two months. She had a history of extraction of mobile maxillary left posterior teeth two months prior, after which she experienced compromised wound healing. The patient reported functional difficulties, including mastication, that had developed over the same timeframe. Her medical history included diabetes for which she had been under medication for the past two years. Intraoral examination revealed a bony defect measuring approximately 3x1.5cm in the edentulous area corresponding to the regions of the second premolar to the first molar (24, 25, 26). This defect extended anteroposteriorly from the region of the second premolar to the mesial aspect of the second molar (27 region) and mediolaterally from the edentulous alveolar ridge to the buccal vestibule. The defect's floor was covered with pseudomembranous slough. Palpation elicited tenderness and spontaneous bleeding, with no pus discharge observed (Figure 2A). A provisional diagnosis of chronic osteomyelitis associated with the 24, 25, 26 region was made, with mucormycosis considered as a differential diagnosis.
The patient underwent antibiotic sensitivity testing, which detected mild species of Klebsiella pneumoniae and Pseudomonas aeruginosa. Biochemical investigations revealed a fasting blood glucose level of 137 mg/dL and a postprandial blood glucose level of 229 mg/dL. Radiographic assessments included an orthopantomogram (OPG), which showed erosion of the alveolar process of the maxilla in the partially edentulous 24, 25, 26 region, displaying a moth-eaten appearance (Figure 2B). Computed Tomography (CT) scans indicated bone erosion with a similar appearance in the regions from the central incisor to the first molar (21 to 26 regions), including perforation of both buccal and palatal cortical plates. A hypodense area containing hyperdense foci (sequestra) was observed, alongside mucosal thickening of the left maxillary sinus (Figure 2C).
Surgical debridement and curettage of the necrotic bone and sequestrum were performed under local anesthesia. Histopathological analysis revealed multiple fragments of bone trabeculae and sequestrum, with large areas of necrotic debris accompanied by neutrophils and lymphoplasmacytic infiltration. The surface of the trabeculae exhibited irregular areas of resorption and empty lacunae, with no evidence of granuloma or atypia. These findings were indicative of chronic sclerosing osteomyelitis in the 24, 25, 26 region. Postoperatively, the patient was prescribed oral antibiotics, including Ofloxacin 200 mg twice daily and Metronidazole 400 mg thrice daily, for a duration of 5 days.
Case Report 3
A 40-year-old male presented to the department of oral medicine, reporting recurrent pain and swelling on the left side of his face over the last four months. Initially, symptoms were intermittent and temporarily alleviated by self-administered medication, but over the last month, the patient experienced constant discomfort accompanied by restricted mouth opening and pus discharge. This patient had a history of an incomplete extraction of tooth 37 one year prior, with the remaining fragment removed six months later. Additionally, he reported a 10-year history of cigarette smoking, mawa chewing, and alcohol consumption but no significant systemic illnesses.
Clinical examination revealed a single diffuse swelling in the left parasymphysis region, approximately 3.5 x 3 cm in size, with a dry necrotic scab overlying the surface and pus discharge evident through a sinus opening (Figure 3A). The swelling was tender and firm. Intraorally, a similar diffuse swelling, approximately 3 x 2.5 cm, affected the left buccal vestibular region corresponding to teeth 34, 35, 36, and 37, which was also tender and firm (Figure 3B). No intraoral sinus opening or pus discharge was observed here. Tooth 36 demonstrated grade II mobility. Pulp vitality tests indicated that teeth 35 and 36 were non-vital.
Based on these findings, a provisional diagnosis of osteomyelitis associated with teeth 35 and 36 was made. Radiographic evaluation included Orthopantomogram (OPG) and Cone Beam Computed Tomography (CBCT). The OPG identified a single diffuse mixed lesion with a poorly defined radiolucent border surrounding the periapical region of teeth 35 and 36 (Figure 3C). CBCT imaging revealed a single diffuse hypodense area with hyperdense foci in the periapical region of teeth 35 and 36, measuring approximately 1.9 x 1.4 x 2.3 cm, including buccal cortical plate perforation in the region of tooth 36, consistent with osteomyelitis (Figure 3D).
Antibiotic sensitivity testing identified the causative agent as Methicillin-Sensitive Coagulase Negative Staphylococcus species, which showed resistance to co-trimoxazole and penicillin, but sensitivity to ciprofloxacin, clindamycin, doxycycline, erythromycin, ofloxacin, rifampicin, and tetracycline. The patient underwent the extraction of teeth 36 and 35 followed by bone curettage (Figure 3E). Histopathological examination of the curetted bone confirmed the diagnosis of chronic osteomyelitis, showing numerous sclerotic and necrotic bone spicules within the marrow spaces, which also contained few bacterial colonies. Irregular areas of resorption and empty lacunae were observed on the surface of the trabeculae.
The patient was prescribed a combination of cefixime 200mg and ofloxacin 200mg, along with metronidazole 400mg for five days, as part of the treatment plan.
Prognostic Management and Outcomes in Case Studies
In the case studies under review, the primary management strategy involved confronting prognostic challenges through the application of targeted antibiotic therapy, determined by sensitivity testing, and addressing associated risk factors. The initial case demonstrated a favorable prognosis after one month, characterized by satisfactory wound healing and the absence of lesion recurrence as depicted in Figure 4.
In the third case, involving a male patient, the follow-up two months post-operation revealed complete healing of the sinus opening wound and, intraorally, satisfactory progress in wound healing adjacent to teeth 35 and 36. The post-operative orthopantomogram (OPG) confirmed the improvement in prognosis, exhibiting no pathological radiological signs and the presence of saucer-shaped surgical bony defects in the areas surrounding teeth 35 and 36, shown in Figure 5.
In the first two cases, uncontrolled diabetes emerged as a significant risk factor, necessitating an integrated approach that included consultations with diabetologists. This multidisciplinary effort succeeded in stabilizing blood sugar levels, leading to positive outcomes in wound healing after surgical interventions. Conversely, the third case involved a patient whose chronic infection was complicated by a history of smoking and alcohol use. In addition to antibiotic treatment, the patient was advised on tobacco cessation. Following the surgical procedure, wound healing proceeded without complications, and no instances of reinfection were observed during a follow-up period of one month.
| HALLMARKS OF OSTEOMYELITIS |
|---|
| Infectious stimuli with predisposing factors |
| Hyperemia of the tissues |
| Tissue necrosis |
| Destruction of bacteria by cytokines |
| Vascular thrombosis |
| Pus formation and accumulation |
| Vascular collapse |
| Invasion of pus into the haversian and nutrient canal |
| Periosteal reaction |
| Elevation of cortex |
| Compromised vascular supply |
| Pus invading the periosteum |
| Mucosal fistula |
DISCUSSION
Chronic osteomyelitis is an inflammatory bone condition characterized by necrosis within mineralized and marrow tissues. Its predisposition hinges on the virulence factors of the causative organism and the host's defense mechanisms4. Common risk factors are depicted in Figure 6.
A retrospective study conducted by Ramita et al. in 2020 explored demographic variations and underlying causes among 27 individuals with a specific condition. The study found that 20 participants had identifiable systemic causes, with 13 reporting a history of substance abuse. Additionally, a notable link was identified between maxillary osteomyelitis and concurrent medical conditions5. Another aspect of the discussion is the segregation of osteomyelitis hallmarks, as detailed in Table 1.
In the context of chronic inflammation, bone necrosis leads to the formation of a sequestrum, surrounded by a sheath of new bone development known as involucrum, within granulation tissue beds6.
The cornerstone of managing osteomyelitis lies in comprehensive history-taking and clinical examinations. These foundational steps are augmented by pus culture sensitivity and antibiotic susceptibility tests, crucial for identifying pathogens, guiding treatment, and averting sepsis7. However, Lakshmi Velu et al. emphasized the importance of specific microbiologic specimens over wound swabs for accurate chronic osteomyelitis diagnosis, citing biopsies or tissue specimens as yielding more dependable results. This study also identified Staphylococcus aureus as the predominant agent, uncovering significant antibiotic resistance in Gram-negative bacilli8. Additionally, Zhang et al. demonstrated the advantages of metagenomic next-generation sequencing (mNGS) over traditional culture methods in osteomyelitis microbiological analysis, showcasing its ability to detect anaerobic bacteria, Mycobacterium tuberculosis, and mixed infections more effectively9.
The visualization of chronic osteomyelitis features utilizes various radiographic techniques ranging from basic radiolucency to complex patterns of mixed radiopacity and radiolucency with periosteal bone reactions. Preferred imaging modalities include intraoral periapical radiographs, orthopantomography, occlusal imaging, and computed tomography (CT), although conventional X-rays are limited by their inability to detect changes until approximately 40% of healthy bone demineralization has occurred10. Bone scintigraphy and newer imaging technologies like cone-beam CT offer advanced capabilities for depicting bony defects and other characteristics critical to osteomyelitis diagnosis10.
Effective management of osteomyelitis encompasses early diagnosis, pus drainage, bacteriological culture and sensitivity analysis, appropriate antibiotic therapy, supportive treatments, surgical debridement, and reconstruction as needed, alongside adjunctive hyperbaric oxygen therapy11. A systematic review indicated the necessity for surgical intervention in chronic mandibular osteomyelitis, ranging from decortication to partial resection, with the use of antibiotic-impregnated beads for sustained infection control12, 13, 14. Pharmacological management primarily involves antibiotics, with some research indicating analgesic benefits, as found in a study by Yoshi et al. regarding roxithromycin15. Rached Lim et al.'s retrospective analysis comparing oral and intravenous antibiotics suggested no notable difference in efficacy, positioning penicillin as an optimal first-line treatment when possible16. The application of antiresorptive agents like bisphosphonates, pamidronate, and denosumab offers promise in chronic osteomyelitis management17, 18. Additionally, nonsteroidal anti-inflammatory drugs (NSAIDs) are integral for pain and inflammation reduction, albeit with careful consideration due to their adverse effect profile12, 19.
Hyperbaric oxygen therapy (HBOT) presents a viable treatment for refractory osteomyelitis cases by delivering 100% oxygen at elevated pressure, enhancing various physiological processes to support healing and increase antibiotic efficacy20. Systematic reviews affirm HBOT's efficacy in refractory jaw osteomyelitis management20.
Conclusions
Timely detection and appropriate antibiotic management of osteomyelitis are crucial for preventing extensive damage to bones and teeth. However, surgical procedures to drain abscesses and remove necrotic bone are often indispensable. Despite advancements in treatment, successful management of osteomyelitis fundamentally depends on a thorough evaluation of both clinical and imaging findings. A comprehensive understanding of the disease's characteristics and continuous monitoring of treatment efficacy in individual cases are essential. Case studies highlight the importance of early diagnosis in effectively treating osteomyelitis while minimizing the need for surgical interventions.
It is imperative for every oral healthcare professional to understand the core principles of osteomyelitis management to avoid serious complications. The collective body of evidence emphasizes the necessity of vigilant clinical monitoring and ongoing evaluation of therapeutic responses to ensure the highest standard of patient care.
Abbreviations
CBCT: Cone Beam Computed Tomography, CT: Computed Tomography, HBOT: Hyperbaric Oxygen Therapy, IOPA: Intraoral Periapical Radiograph, mNGs: metagenomic Next Generation Sequencing, NSAIDS: Non Steroidal Anti Inflammatory Drugs, OPG: Orthopantomogram
Acknowledgments
None.
Author’s contributions
All authors contributed to the study’s conception and design. Saraswathi Gopal K , Narmadha Chandran and Sangavi Ramesh contributed equally to this article as co-first authors. Saraswathi Gopal K, Narmadha chandran and Sangavi Ramesh contributed to undergo the clinical data analysis, diagnostic mapping of the three cases, collect, and interpret the imaging. Preethi Ravi made substantial contributions to collect patient data and clinical data analysis. All authors have read, revised, and approved the final published version of the manuscript. All authors were responsible for submission of our study for publication.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Pincus
D.J.,
Armstrong
M.B.,
Thaller
S.R.,
Osteomyelitis of the craniofacial skeleton. InSeminars in plastic surgery 2009 May.
2009;
23
(2)
:
073-079
.
View Article Google Scholar -
Mehra
H.,
Gupta
S.,
Gupta
H.,
Sinha
V.,
Singh
J.,
Chronic suppurative osteomyelitis of mandible: a case report. Craniomaxillofacial Trauma & Reconstruction.
2013;
6
(3)
:
197-200
.
View Article PubMed Google Scholar -
Malik
S.,
Singh
G.,
Chronic suppurative osteomyelitis of the mandible: A study of 21 cases. Oral Health and Dental Management.
2014;
13
:
971-4
.
-
Schmitt
S.K.,
Osteomyelitis. Infectious Disease Clinics of North America.
2017;
31
(2)
:
325-38
.
View Article PubMed Google Scholar -
Sood
R.,
Gamit
M.,
Shah
N.,
Mansuri
Y.,
Naria
G.,
Maxillofacial osteomyelitis in immunocompromised patients: a demographic retrospective study. Journal of Maxillofacial and Oral Surgery.
2020;
19
(2)
:
273-82
.
View Article PubMed Google Scholar -
Lew
D.P.,
Waldvogel
F.A.,
Osteomyelitis. The New England Journal of Medicine.
1997;
336
(14)
:
999-1007
.
View Article PubMed Google Scholar -
Chen
J.,
Xiong
A.,
Ma
Y.,
Qin
C.,
Ho
C.L.,
Impact of the host-microbiome on osteomyelitis pathogenesis. Frontiers in Molecular Biosciences.
2021;
8
.
View Article PubMed Google Scholar -
Vemu
L.,
Sudhaharan
S.,
Mamidi
N.,
Chavali
P.,
Need for appropriate specimen for microbiology diagnosis of chronic osteomyelitis. Journal of Laboratory Physicians.
2018;
10
(1)
:
021-5
.
View Article Google Scholar -
Zhang
K.,
Bai
Y.Z.,
Liu
C.,
Liu
S.S.,
Lu
X.X.,
Yang
R.G.,
Composition of pathogenic microorganism in chronic osteomyelitis based on metagenomic sequencing and its application value in etiological diagnosis. BMC Microbiology.
2023;
23
(1)
:
313
.
View Article PubMed Google Scholar -
Lee
Y.J.,
Sadigh
S.,
Mankad
K.,
Kapse
N.,
Rajeswaran
G.,
The imaging of osteomyelitis. Quantitative Imaging in Medicine and Surgery.
2016;
6
(2)
:
184-98
.
View Article PubMed Google Scholar -
Birt
M.C.,
Anderson
D.W.,
Bruce Toby
E.,
Wang
J.,
Osteomyelitis: recent advances in pathophysiology and therapeutic strategies. Journal of Orthopaedics.
2016;
14
(1)
:
45-52
.
View Article PubMed Google Scholar -
Timme
M.,
Bohner
L.,
Huss
S.,
Kleinheinz
J.,
Hanisch
M.,
Response of different treatment protocols to treat chronic non-bacterial osteomyelitis (CNO) of the mandible in adult patients: a systematic review. International Journal of Environmental Research and Public Health.
2020;
17
(5)
:
1737
.
View Article PubMed Google Scholar -
Zimmerli
W.,
Osteomyelitis therapy: antibiotic therapy. InOsteomyelitis of the JawsSpringer Berlin Heidelberg: Berlin, Heidelberg; 2009.
View Article Google Scholar -
Khansa
I.,
Barker
J.C.,
Ghatak
P.D.,
Sen
C.K.,
Gordillo
G.M.,
Use of antibiotic impregnated resorbable beads reduces pressure ulcer recurrence: A retrospective analysis. Wound Repair and Regeneration.
2018;
26
(2)
:
221-7
.
View Article PubMed Google Scholar -
Yoshii
T.,
Nishimura
H.,
Yoshikawa
T.,
Furudoi
S.,
Yoshioka
A.,
Takenono
I.,
Therapeutic possibilities of long-term roxithromycin treatment for chronic diffuse sclerosing osteomyelitis of the mandible. The Journal of Antimicrobial Chemotherapy.
2001;
47
(5)
:
631-7
.
View Article PubMed Google Scholar -
Lim
R.,
Mills
C.,
Burke
A.B.,
Dhanireddy
S.,
Beieler
A.,
Dillon
J.K.,
Are oral antibiotics an effective alternative to intravenous antibiotics in treatment of osteomyelitis of the jaw?. Journal of Oral and Maxillofacial Surgery.
2021;
79
(9)
:
1882-90
.
View Article PubMed Google Scholar -
Kuroshima
S.,
Sasaki
M.,
Sawase
T.,
Medication-related osteonecrosis of the jaw: A literature review. Journal of Oral Biosciences.
2019;
61
(2)
:
99-104
.
View Article PubMed Google Scholar -
Otto
S.,
Burian
E.,
Troeltzsch
M.,
Kaeppler
G.,
Ehrenfeld
M.,
Denosumab as a potential treatment alternative for patients suffering from diffuse sclerosing osteomyelitis of the mandible-A rapid communication. Journal of Cranio-Maxillo-Facial Surgery.
2018;
46
(4)
:
534-7
.
View Article PubMed Google Scholar -
Kim
S.M.,
Lee
S.K.,
Chronic non-bacterial osteomyelitis in the jaw. Journal of the Korean Association of Oral and Maxillofacial Surgeons.
2019;
45
(2)
:
68-75
.
View Article PubMed Google Scholar -
Savvidou
O.D.,
Kaspiris
A.,
Bolia
I.K.,
Chloros
G.D.,
Goumenos
S.D.,
Papagelopoulos
P.J.,
Effectiveness of hyperbaric oxygen therapy for the management of chronic osteomyelitis: a systematic review of the literature. Orthopedics.
2018;
41
(4)
:
193-9
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 5 (2024)
Page No.: 6474-6481
Published on: 2024-05-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 4294 times
- PDF downloaded - 1212 times
- XML downloaded - 111 times
 Biomedpress
Biomedpress







