Abstract
Skin aging, influenced by both intrinsic and extrinsic factors, leads to structural and functional deterioration characterized by wrinkles, reduced elasticity, and impaired wound healing. Mesenchymal stem cell-derived exosomes (MSC-exos) have emerged as a promising therapeutic option, offering multifaceted benefits for skin rejuvenation. These nano-sized extracellular vesicles exhibit exceptional bioavailability, biocompatibility, and immunomodulatory properties, addressing challenges associated with conventional treatments. MSC-exos enhance collagen synthesis, modulate inflammation, and promote angiogenesis through molecular pathways such as PI3K/Akt and Notch signaling. Furthermore, their ability to deliver bioactive molecules precisely to target cells underscores their therapeutic potential in skin repair and anti-aging applications. However, challenges remain regarding large-scale production, targeting efficiency, and regulatory frameworks, warranting further research to translate these innovative therapies into clinical practice.
Introduction
Aging of the skin is a dynamic and multifactorial process influenced by genetic predispositions, hormonal shifts, and external stressors such as UV exposure, environmental pollutants, and lifestyle behaviors. This progression manifests as structural degradation, including wrinkles, pigmentation changes, and reduced elasticity, alongside diminished regenerative capacities, such as impaired wound healing. While existing anti-aging therapies—ranging from topical formulations to minimally invasive interventions—have achieved varying degrees of success, their effects are often transient and target isolated symptoms rather than the underlying biological mechanisms.
Emerging advancements in regenerative medicine, particularly the application of MSC-exos, offer a groundbreaking avenue for skin rejuvenation. These nanoscale vesicles, derived from stem cells, demonstrate remarkable potential to rejuvenate aged skin by orchestrating collagen remodeling, mitigating chronic inflammation, and stimulating angiogenesis. Unlike traditional approaches, MSC-exos provide a holistic solution, addressing both the causes and manifestations of skin aging.
This review delves into the transformative potential of MSC-derived exosomes in combating skin aging. By examining their unique bioavailability, immunomodulatory properties, and effects on collagen and vascular networks, we aim to highlight their promise as a next-generation therapy. Additionally, the challenges surrounding large-scale production, delivery precision, and regulatory barriers are discussed, offering insights into the future of this innovative field.
Overview of Skin Aging
The skin, the largest organ of the human body, covers an area of 2 m² and represents approximately 15% of the total body weight in adults. Its thickness ranges from 0.1 mm at its thinnest to 1.5 mm at its thickest1, 2. Premature photoaged skin is characterized by various features, including a thickened epidermis, mottled discoloration, deep wrinkles, laxity, dullness, and a rough texture3, 4. A prominent manifestation of aging is skin sagging, which occurs due to the gradual loss of elasticity5, 6. In older adults, the rate of epidermal turnover and cell desquamation slows down, influencing the timing of aesthetic treatments. Accelerating the cell cycle has been shown to improve skin appearance and enhance wound healing, making these effects important targets for various products and procedures. The weakening of the dermal-epidermal junction in extrinsically aged skin may also contribute to wrinkle formation due to the loss of fibrillin-positive structures and a decrease in collagen type VII content7, 8, 9. Solar elastosis, commonly seen in the sun-exposed skin of the elderly, results from collagen breakdown by matrix metalloproteinases, serine proteases, and other proteases, which increase collagen degradation in photoaged skin10, 11. As skin ages, the ratio of type III to type I collagen increases due to a decline in collagen levels12, 13. Collagen content decreases by approximately 1% per year per unit area of skin14. Numerous studies available on PubMed have explored the impact of factors such as oxidative stress, ultraviolet (UV) radiation, and inflammation on skin aging15. These studies indicate that these factors contribute to the degeneration of collagen and other extracellular matrix (ECM) components, leading to wrinkles and loss of skin elasticity. The skin has a natural ability to heal itself, involving a cascade of processes such as hemostasis, inflammation, proliferation, and tissue remodeling. However, when this healing process is interrupted, altered, or prolonged, wound healing may be delayed, or chronic wounds may develop.
Limitations of Current Therapeutic Strategies for Skin Aging
Skin aging is a complex process influenced by both intrinsic and extrinsic factors, characterized by wrinkles, fine lines, irregular pigmentation, and a progressive loss of skin elasticity, firmness, and moisture. Given the increasing demand for effective anti-aging treatments, recent years have seen significant advancements in therapeutic approaches to address skin aging.
One widely adopted strategy involves the use of topical medicines that target specific aging pathways. These include antioxidants, retinoids, and alpha-hydroxy acids. Retinoids, derivatives of vitamin A, enhance collagen synthesis and reduce the appearance of wrinkles and fine lines. Alpha-hydroxy acids, such as glycolic acid and lactic acid, exfoliate the skin while promoting collagen production16, 17, 18. Antioxidants like vitamins C and E protect the skin from oxidative damage caused by UV rays and environmental toxins19, 20, 21.
Minimally invasive approaches, such as injectable fillers and botulinum toxin (Botox) injections, are also commonly employed. Injectable fillers, including hyaluronic acid and calcium hydroxyapatite, restore skin volume and reduce the visibility of wrinkles22, 23, 24. Botox injections relax the facial muscles responsible for dynamic wrinkles, particularly around the eyes and forehead25, 26, 27.
More invasive procedures, such as chemical peels and laser resurfacing, target deeper layers of the skin to stimulate collagen synthesis. Chemical peels are effective for treating acne scars and sun damage28, while laser resurfacing is particularly useful for addressing uneven pigmentation and deeper wrinkles29.
Emerging regenerative medicine approaches have garnered attention for their potential to repair and regenerate aged skin tissues. Stem cells and growth factors are central to these strategies. Stem cells possess the capacity to differentiate into a myriad of cellular phenotypes, encompassing dermal cells, and thereby facilitating the replacement of compromised or senescent cells. Growth factors, such as platelet-rich plasma, stimulate collagen production and ECM remodeling, facilitating tissue repair30, 31.
Among alternative treatments, exosome-based therapies are gaining traction due to their unique advantages. Exosomes, tiny vesicles released by cells, including stem cells, contain bioactive compounds like growth factors, cytokines, and microRNAs that aid in tissue repair and regeneration32, 33, 34. A key advantage of exosomes is their non-immunogenic nature, minimizing the risk of adverse immune reactions, unlike injectable fillers or other invasive procedures35, 36.
Exosomes also address multiple aspects of skin aging simultaneously. They enhance cell proliferation and differentiation37, boost collagen synthesis38, 39, and reduce inflammation40, 41, all of which are critical for skin regeneration. Furthermore, exosomes can be efficiently isolated from various cell types, making them a promising therapeutic option. Preclinical research has shown that exosomes can enhance wound healing and stimulate tissue regeneration, as evidenced by studies on animal models and in vitro experiments using human skin cells42, 43, 44, 45.
Exosomes and their potential in dermatology
Exosomes are nano-sized biovesicles released into surrounding body fluids when multivesicular bodies fuse with the plasma membrane39. These vesicles originate from the internal folding of endosomal membranes, resulting in the formation of intraluminal vesicles, which are then secreted as exosomes. Acting as mediators of intercellular communication, exosomes transfer their cargo to target cells or activate signaling pathways on the cell surface. They play essential roles in physiological and pathological processes, including immune responses, cell proliferation, tissue homeostasis, cancer, and neurodegenerative diseases39.
Exosomes contain a wide variety of biomolecules sourced from their parent cells, such as proteins, lipids, nucleic acids, and carbohydrates46. Their protein cargo comprises functional categories such as tetraspanins, heat shock proteins, and cytoskeletal proteins. Lipids, like sphingomyelin and cholesterol, are enriched in exosomes, contributing to their structural integrity and biological functions. Nucleic acids in exosomes, including messenger RNAs, long non-coding RNAs, and microRNAs, have the ability to modulate gene expression in recipient cells47, 48.
Exosomes impact a range of cellular processes, including cell growth, differentiation, and apoptosis39, 46. They also play a dual role in immune regulation, either stimulating or inhibiting immune responses depending on the context49, 50. In addition, exosomes are involved in the progression of various diseases, such as cancer, cardiovascular diseases, and neurodegenerative disorders, underscoring their potential for diagnostic and therapeutic applications51.
In regenerative medicine, exosomes derived from MSC-exos offer significant advantages over traditional live stem cell therapies. While adipose-derived stem cells (ADSCs) have shown limited efficacy due to apoptosis shortly after transplantation52, 53 and challenges related to circulation and thrombus formation54, 55, MSC-exos mitigate these issues. Intravenous injection of MSCs may cause aggregation in microcirculation and pose risks of mutagenicity or oncogenicity, risks that are absent with MSC-exos. Additionally, MSC-exos remain stable during long-term storage, facilitating safe transport and delayed therapeutic application.
The potential of exosomes in treating skin abnormalities has been extensively explored in recent years. Their primary advantages include stability, resistance to immunological rejection, and the capacity to directly stimulate target cells. Unlike conventional treatments, exosomes can exert multiple therapeutic effects through a single component, making them a versatile and promising option for clinical applications.
MSC-Derived Exosomes in Skin Aging Treatment
Bioavailability and Delivery Mechanisms
Exosomes, derived from the late endocytic compartment, diffuse easily into intracellular fluids and rapidly fuse with target cells, enhancing their potential for therapeutic delivery. They exhibit exceptional interaction with cellular membranes, which is crucial for efficient drug delivery. Recent in vivo studies have shown that exosomes exhibit specific cell tropism, guiding them to disease-affected tissues and organs56. This targeting ability is a result of both their intrinsic properties and engineered modifications. Exosomes express unique surface proteins that enhance their natural ability to bind to specific cell types, facilitating efficient targeting and drug delivery57.
The lipid bilayer structure of exosomes contributes to their low immunogenicity, allowing prolonged circulation and reducing the risk of immune rejection. Their natural structure also helps maintain integrity, ensuring that they protect their cargo until reaching the target site58, 59, 60. Furthermore, exosomes are biodegradable, reducing long-term toxicity risks compared to synthetic carriers that may persist in the body and cause chronic inflammation61, 62. Genetic modifications can enhance the targeting capabilities of exosomes. Incorporating homing peptides and ligands allows exosomes to be directed to specific organs or tissues, improving therapeutic efficacy59. These modifications can also facilitate on-demand drug release in response to specific stimuli, enhancing precision in drug delivery58. Additionally, surface proteins such as tetraspanins and integrins improve exosomes' natural targeting ability, ensuring efficient delivery to the intended tissues57. Engineered exosomes can also express chemokine receptors to enhance their tropism toward inflamed tissues, which has been demonstrated in treatments for conditions like atherosclerosis63. This ability is further supported by transcytosis, a process enhanced by factors such as inflammation64.
Despite their promise, exosomes' targeting efficiency can be inconsistent, and their production remains challenging. Synthetic alternatives may offer more controlled delivery mechanisms, highlighting the need for further optimization of exosome engineering60. Exosome production involves extensive cell culture and purification processes, which are resource-intensive and time-consuming. Variability in exosome composition requires rigorous quality assessment to ensure therapeutic efficacy65, 66. Additionally, the lack of standardized manufacturing guidelines is a significant barrier67. The regulatory landscape for exosome-based therapies is complex, varying by jurisdiction, and requires comprehensive pharmacokinetic and therapeutic efficacy data, along with toxicology studies and potency assays to ensure safety and efficacy in clinical settings68. Low yield and batch reproducibility further limit their scalability, posing another challenge for widespread clinical application. While the potential of exosomes as therapeutic agents is significant, these challenges must be addressed through ongoing research. Optimization of production methods, improvements in targeting precision, and regulatory advancements are crucial to unlocking the full therapeutic potential of exosomes.
Low Immunogenicity and Safety Profile
MSC-exos have attracted considerable interest due to their immunomodulatory and regenerative capabilities. They increase Treg production in vivo and in vitro through TGF-α and IFN-γ69.
MSC-exos regulate pattern recognition receptors (PRRs), modulate B-cell activities, polarize macrophages toward anti-inflammatory phenotypes, and fine-tune T-cell activity. These processes orchestrate diverse immunological responses, making MSC-exos valuable for precision medicine and therapeutic interventions70.
MSC-exos primarily induce M2-like macrophage polarization through CD73 activity, which converts AMP to adenosine. This adenosine activates A2A and A2B receptors, triggering AKT/ERK signaling pathways that alleviate inflammation and immune dysfunction71. Furthermore, MSC-exos pre-treated in a diabetic environment (Exo-pre) enhance M2 polarization via miR-486-5p, which targets PIK3R1 and modulates the PI3K/Akt pathway72.
Additionally, miR-150-5p within MSC-exos downregulates the PI3K/Akt/mTOR pathway by targeting Irs1 in recipient macrophages, promoting M2 polarization and inhibiting M1 activation73. They also inhibit LPS-induced inflammatory responses, increase IL-10 and Arg-1 levels, enhance CD206 expression, reduce NF-κB signaling, and stimulate STAT3 activity, further supporting M2 macrophage polarization74. In vivo studies show MSC-derived extracellular vesicles enhance M2 macrophage polarization, providing a novel therapeutic strategy to mitigate inflammatory conditions75.
MSC-exos modulate T-cell activity by decreasing T-cell proliferation and Th1 differentiation while promoting Treg differentiation and restoring the Th17/Treg balance. These effects are mediated through pathways involving TGF-β and autophagy, as demonstrated in studies on primary Sjögren's syndrome76, 77. MSC-exos also promote the generation of IFN-γ+/Foxp3+ T cells with suppressive capacity and influence Th1 metabolism. Proteins such as p27kip1 and Cdk2 play significant roles in cell cycle arrest and T-cell suppression mediated by MSC-exos78. Moreover, exosomes enriched with CD73 can inhibit T-cell proliferation, modulate T-cell differentiation, and enhance immunosuppressive effects, making them potential therapeutic agents for autoimmune diseases79.
MSC-exos reduce neutrophil infiltration, attenuate NLRP3 inflammasome activation, and suppress the formation of neutrophil extracellular traps (NETs). They achieve this by up-regulating miR-199 in neutrophils, thereby decreasing NETs expression after stimulation80.
Effects on Collagen Synthesis and Remodeling
Managing wound healing is a complex process involving sequential, overlapping stages, where disruptions can lead to chronic, non-healing wounds. Collagen, a critical component of the ECM in the dermis, provides structural integrity and support to the skin81. In aging skin, collagen type I and elastic fibers become fragmented, causing dermal layer damage and impairing skin elasticity82, 83. Reconstructing the dermal structure can potentially mitigate aging effects and improve wound healing.
Several treatment options have been explored to counteract collagen degradation. Topical agents such as retinoids, alpha-hydroxy acids, and antioxidants stimulate collagen synthesis and inhibit its breakdown, but their effects are often limited and require prolonged use84, 85, 86. Invasive procedures like laser resurfacing, micro-needling, and injectable fillers can improve skin texture and reduce wrinkles. However, these methods carry risks such as scarring and infection84, 87, 88, 89, 90, 91.
Exosomes, nano-sized extracellular vesicles, offer a promising alternative. They can be applied topically or injected into the skin, allowing precise delivery to the dermis while minimizing systemic side effects. Exosomes contain bioactive molecules, including growth factors and cytokines, which promote angiogenesis and tissue remodeling92, 91, 93. Derived from various cell types, exosomes can influence collagen metabolism by breaching the epidermal barrier and interacting with target cells to regulate ECM homeostasis94, 95. MSC-exosomes exhibit remarkable capability in delivering functional molecules, including proteins, lipids, mRNAs, and miRNAs, to recipient cells. Their phospholipid bilayer enables efficient delivery, influencing cellular processes like gene regulation, immune modulation, and tissue repair96, 97, 98, 99. MSC-exosomes can enhance collagen production and reduce breakdown, particularly in fibroblasts, leading to smoother, more elastic skin95, 100, 101, 102, 103. They also address oxidative stress and inflammation, major contributors to skin aging104, 105.
MSC-exosomes are a promising strategy for treating skin disorders characterized by collagen dysregulation. These exosomes regulate collagen synthesis and degradation in fibroblasts by modulating the expression of key genes and pathways. For instance, they enhance the expression of collagen-related genes such as col1a1 while reducing the levels of matrix metalloproteinases (MMPs) and increasing the expression of tissue inhibitors of metalloproteinases (TIMPs), thereby preserving the balance of the ECM100, 106. Furthermore, MSC-exosomes contain miRNAs, such as miR-34b-3p and miR-144-3p, which regulate fibroblast behavior and collagen metabolism through pathways like PI3K/Akt 107, 108, 109.
During the early stages of wound healing, MSC-exosomes enhance type I and III collagen formation, promoting effective tissue regeneration. In later stages, they limit excessive collagen deposition, reducing scar formation. For instance, ADSC-derived exosomes adjust the type III-to-type I collagen ratio, aiding in balanced tissue repair110, 111. Exosomes rich in miR-21-5p and miR-125b-5p suppress TGF-β receptors, preventing myofibroblast differentiation and promoting better skin regeneration112. Additionally, UCB-MSC-exos inhibit collagen I production while encouraging skin cell proliferation and migration, further optimizing wound healing outcomes.
Promotion of Angiogenesis
Aging induces significant morphological and functional changes in cutaneous blood vessels, impacting overall skin health and vascular functionality. Structural remodeling includes vascular adventitia thickening and a decrease in the density of skin lymphatic vessels, impairing fluid transport and immune responses113, 114. Functional decline manifests through reduced vasomotor function, increased blood viscosity, and heightened platelet aggregation, compromising vascular health. Furthermore, aging diminishes the ability of blood vessels to respond to stimuli, evidenced by reduced skin blood flow and impaired post-occlusive hyperemia115.
Endothelial dysfunction in aging cutaneous blood vessels is marked by reduced nitric oxide (NO) production, increased oxidative stress, and impaired vasodilation. Aging reduces NO bioavailability due to heightened superoxide anion production, leading to vascular dysfunction116, 117. This dysfunction affects nutrient delivery and waste removal in skin tissues, contributing to skin sagging, dryness, and wrinkle formation, all hallmarks of declining skin health116, 118 . Increased vascular stiffness further exacerbates these conditions, reflecting overall skin aging.
Exosomes, especially those originating from MSCs and endothelial cells, play a key role in angiogenesis. They transport pro-angiogenic factors, including VEGF and bFGF, to target cells, thereby stimulating endothelial cell proliferation, migration, and the formation of blood vessels119, 120. Both in vivo and in vitro studies show that exosomes derived from MSCs promote wound healing and angiogenesis. This is evident in diabetic skin ulcer models, where they accelerate wound closure and the formation of new blood vessels121, 122, 123.
MSC-derived exosomes promote angiogenesis and improve skin health through various molecular pathways. Exosomal miR-126 activates the PI3K/Akt signaling pathway, enhancing VEGF and angiopoietin-1 expression, thereby stimulating endothelial cell proliferation and migration124, 125. Exosomal miR-17-92 further supports angiogenesis by inhibiting ferroptosis and enhancing endothelial cell functions126. MicroRNA-125a, found in adipose-derived MSC exosomes, suppresses the angiogenic inhibitor DLL4, promoting the formation of endothelial tip cells120.
MSC-derived exosomes exhibit anti-aging effects on skin vasculature by regulating angiogenic factors and improving collagen production. These exosomes stimulate dermal fibroblast proliferation, migration, and collagen deposition, facilitating tissue regeneration and wound healing127. Additionally, exosomal Jagged1, derived from HIF-1α-overexpressing MSCs, enhances angiogenesis via Notch signaling activation in endothelial cells, further supporting vascular rejuvenation128.
Anti-inflammatory and Immunomodulatory Effects
Chronic inflammation significantly contributes to skin aging through mechanisms often referred to as "inflammaging." This persistent low-grade inflammation accelerates cellular senescence and disrupts skin homeostasis. The accumulation of senescent cells triggers the secretion of pro-inflammatory factors, known as the senescence-associated secretory phenotype, which perpetuates inflammation and induces further senescence in neighboring cells129, 130. Aging is also associated with immunosenescence, a decline in the immune system's ability to manage inflammation, leading to increased senescent cells and inflammatory mediators131. External factors like UV radiation and pollutants exacerbate this process, with UVB-induced inflammation being mediated by molecules such as nitric oxide, prostaglandin E2, and cytokines like IL-1 and IL-6, predominantly regulated by NF-κB in keratinocytes132.
Chronic inflammation disrupts epidermal balance, leading to common aging features like skin thinning and a weakened barrier [3]. Pro-inflammatory cytokines, such as IL-1β and TNF-α, stimulate the production of matrix metalloproteinases (MMPs) and cathepsins, enzymes that break down the ECM, particularly collagen. This ECM breakdown results in reduced skin elasticity and wrinkle formation133, 134. Advanced glycation end products (AGEs) further contribute to skin dysfunction by triggering oxidative stress, disrupting collagen and elastin, and amplifying inflammation through reactive oxygen species (ROS)135, 136, 137.
Exosomes, which are small extracellular vesicles, are crucial in regulating inflammation by promoting communication between immune cells. Exosomes released from LPS-preconditioned MSCs can alter macrophage polarization, steering it towards an anti-inflammatory M2 phenotype through the NF-κB/NLRP3 signaling pathway138. Furthermore, engineered exosomes loaded with anti-inflammatory agents, such as curcumin, show potential in treating inflammation-related conditions, including rheumatoid arthritis and spinal cord injuries139. Modifications like hyaluronic acid or polyethylene glycol enhance exosome targeting and therapeutic efficacy138.
MSC-Exos exhibit significant anti-inflammatory effects, outperforming exosomes from other cell types. These vesicles are enriched with microRNAs, proteins, and cytokines that modulate immune responses and reduce inflammation140, 141. In skin aging, MSC-Exos downregulate pro-inflammatory cytokines like IL-1β and TNF-α and inhibit the NF-κB pathway, mitigating chronic inflammation and oxidative stress while promoting cell survival19, 20. Additionally, MSC-Exos enhance protective proteins like SIRT1 and P53, improving skin cell viability under stress conditions142.
The anti-inflammatory potential of adipose-derived stem cell exosomes (ADSC-Exos) has shown promise in treating atopic dermatitis. ADSC-Exos notably reduce the levels of pro-inflammatory cytokines, including IL-4, IL-13, and TNF-α, by 30-50%, in a dose-dependent fashion143, 144. Clinical studies demonstrate their efficacy in reducing erythema, improving skin hydration, and lowering clinical scores over 12 weeks143. MSC-Exos similarly reduce inflammatory cytokines like TNF-α and IL-17 in aged skin, inhibiting pathways such as STAT3 and suppressing dendritic cell activation, contributing to a balanced immune environment145, 146.
Conclusions
MSC-derived exosomes represent a revolutionary approach to combating skin aging, integrating regenerative and immunomodulatory capabilities into a single therapeutic platform. Their ability to modulate collagen remodeling, reduce inflammation, and enhance vascularization positions them as superior to traditional treatments in both efficacy and safety. Despite these advantages, significant obstacles, including manufacturing scalability, quality control, and regulatory hurdles, must be overcome to facilitate widespread clinical adoption. Future research should focus on optimizing exosome engineering, improving delivery mechanisms, and standardizing therapeutic protocols to unlock the full potential of this innovative treatment for skin aging.
Abbreviations
ADSCs: Adipose-Derived Stem Cells, AKT/ERK: Protein Kinase B / Extracellular Signal-Regulated Kinase, AMP: Adenosine Monophosphate, DLL4: Delta-like Ligand 4, ECM: Extracellular Matrix, IFN-γ: Interferon-gammam, iRNAs: MicroRNAs, MMPs: Matrix Metalloproteinases, MSC-exos: Mesenchymal Stem Cell-derived Exosomes, NETs: Neutrophil Extracellular Traps, NF-κB: Nuclear Factor Kappa B, PI3K/Akt: Phosphoinositide 3-Kinase / Protein Kinase B, PRRs: Pattern-Recognition Receptors, ROS: Reactive Oxygen Species, SIRT1: Silent Information Regulator T1, STAT3: Signal Transducer and Activator of Transcription 3, TGF-α: Transforming Growth Factor-alpha, Th1/Th17/Treg: T-helper cells type 1 / type 17 / Regulatory T-cells, TIMPs: Tissue Inhibitors of Metalloproteinases, UCB-MSC: Umbilical Cord Blood-Mesenchymal Stem Cells
Acknowledgments
None.
Author’s contributions
Not applicable.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Nedelec
B.,
Forget
N.J.,
Hurtubise
T.,
Cimino
S.,
de Muszka
F.,
Legault
A.,
Skin characteristics: normative data for elasticity, erythema, melanin, and thickness at 16 different anatomical locations. Skin Research and Technology.
2016;
22
(3)
:
263-75
.
View Article PubMed Google Scholar -
Osmancevic
A.,
Gillstedt
M.,
Landin-Wilhelmsen
K.,
Wennberg Larkö
A.M.,
Larkö
O.,
Holick
M.F.,
Size of the exposed body surface area, skin erythema and body mass index predict skin production of vitamin D. Journal of Photochemistry and Photobiology. B, Biology.
2015;
149
:
224-9
.
View Article PubMed Google Scholar -
Green
A.C.,
Hughes
M.C.,
McBride
P.,
Fourtanier
A.,
Factors associated with premature skin aging (photoaging) before the age of 55: a population-based study. Dermatology (Basel, Switzerland).
2011;
222
(1)
:
74-80
.
View Article PubMed Google Scholar -
Sachs
D.L.,
Varani
J.,
Chubb
H.,
Fligiel
S.E.,
Cui
Y.,
Calderone
K.,
Atrophic and hypertrophic photoaging: Clinical, histologic, and molecular features of 2 distinct phenotypes of photoaged skin. Journal of the American Academy of Dermatology.
2019;
81
(2)
:
480-8
.
View Article PubMed Google Scholar -
Chaudhary
M.,
Khan
A.,
Gupta
M.,
Skin Ageing: Pathophysiology and Current Market Treatment Approaches. Current Aging Science.
2020;
13
(1)
:
22-30
.
View Article PubMed Google Scholar -
Ezure
T.,
Hosoi
J.,
Amano
S.,
Tsuchiya
T.,
Sagging of the cheek is related to skin elasticity, fat mass and mimetic muscle function. Skin Research and Technology.
2009;
15
(3)
:
299-305
.
View Article PubMed Google Scholar -
Bosset
S.,
Barré
P.,
Chalon
A.,
Kurfurst
R.,
Bonté
F.,
André
P.,
Skin ageing: clinical and histopathologic study of permanent and reducible wrinkles. European Journal of Dermatology.
2002;
12
(3)
:
247-52
.
PubMed Google Scholar -
Lavker
R.M.,
Zheng
P.S.,
Dong
G.,
Aged skin: a study by light, transmission electron, and scanning electron microscopy. The Journal of Investigative Dermatology.
1987;
88
(3)
:
44s-51s
.
View Article PubMed Google Scholar -
Langton
A.K.,
Halai
P.,
Griffiths
C.E.,
Sherratt
M.J.,
Watson
R.E.,
The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mechanisms of Ageing and Development.
2016;
156
:
14-6
.
View Article PubMed Google Scholar -
Jackson
R.,
Elderly and sun-affected skin. Distinguishing between changes caused by aging and changes caused by habitual exposure to sun. Canadian Family Physician Medecin de Famille Canadien.
2001;
47
:
1236-43
.
PubMed Google Scholar -
Kim
D.H.,
Oh
G.N.,
Kwon
I.H.,
Seo
S.H.,
Kye
Y.C.,
Ahn
H.H.,
Relationship between skin color and solar elastosis in aged Asian skin: A colorimetric-pathologic correlation. Microscopy Research and Technique.
2017;
80
(10)
:
1073-7
.
View Article PubMed Google Scholar -
Zettersten
E.M.,
Ghadially
R.,
Feingold
K.R.,
Crumrine
D.,
Elias
P.M.,
Optimal ratios of topical stratum corneum lipids improve barrier recovery in chronologically aged skin. Journal of the American Academy of Dermatology.
1997;
37
(3 Pt 1)
:
403-8
.
View Article PubMed Google Scholar -
Varani
J.,
Dame
M.K.,
Rittie
L.,
Fligiel
S.E.,
Kang
S.,
Fisher
G.J.,
Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. American Journal of Pathology.
2006;
168
(6)
:
1861-8
.
View Article PubMed Google Scholar -
Avila Rodríguez
M.I.,
Rodríguez Barroso
L.G.,
Sánchez
M.L.,
Collagen: A review on its sources and potential cosmetic applications. Journal of Cosmetic Dermatology.
2018;
17
(1)
:
20-6
.
View Article PubMed Google Scholar -
de Miranda
R.B.,
Weimer
P.,
Rossi
R.C.,
Effects of hydrolyzed collagen supplementation on skin aging: a systematic review and meta-analysis. International Journal of Dermatology.
2021;
60
(12)
:
1449-61
.
View Article PubMed Google Scholar -
Usuki
A.,
Ohashi
A.,
Sato
H.,
Ochiai
Y.,
Ichihashi
M.,
Funasaka
Y.,
The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Experimental Dermatology.
2003;
12
(s2)
:
43-50
.
View Article PubMed Google Scholar -
Yamamoto
Y.,
Uede
K.,
Yonei
N.,
Kishioka
A.,
Ohtani
T.,
Furukawa
F.,
Effects of alpha-hydroxy acids on the human skin of Japanese subjects: the rationale for chemical peeling. The Journal of Dermatology.
2006;
33
(1)
:
16-22
.
View Article PubMed Google Scholar -
Tang
S.C.,
Yang
J.H.,
Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules (Basel, Switzerland).
2018;
23
(4)
:
863
.
View Article PubMed Google Scholar -
Fernández-García
E.,
Skin protection against UV light by dietary antioxidants. Food {&}amp; Function.
2014;
5
(9)
:
1994-2003
.
View Article PubMed Google Scholar -
Murray
J.C.,
Burch
J.A.,
Streilein
R.D.,
Iannacchione
M.A.,
Hall
R.P.,
Pinnell
S.R.,
A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. Journal of the American Academy of Dermatology.
2008;
59
(3)
:
418-25
.
View Article PubMed Google Scholar -
Eberlein-König
B.,
Ring
J.,
Relevance of vitamins C and E in cutaneous photoprotection. Journal of Cosmetic Dermatology.
2005;
4
(1)
:
4-9
.
View Article PubMed Google Scholar -
Fakih-Gomez
N.,
Kadouch
J.,
Combining Calcium Hydroxylapatite and Hyaluronic Acid Fillers for Aesthetic Indications: Efficacy of an Innovative Hybrid Filler. Aesthetic Plastic Surgery.
2022;
46
(1)
:
373-81
.
View Article PubMed Google Scholar -
Wang
L.L.,
Friedman
O.,
Update on injectables in the nose. Current Opinion in Otolaryngology & Head & Neck Surgery.
2017;
25
(4)
:
307-13
.
View Article PubMed Google Scholar -
Liu
M.H.,
Beynet
D.P.,
Gharavi
N.M.,
Overview of Deep Dermal Fillers. Facial Plastic Surgery.
2019;
35
(3)
:
224-9
.
View Article PubMed Google Scholar -
Maas
C.S.,
Botulinum neurotoxins and injectable fillers: minimally invasive management of the aging upper face. Otolaryngologic Clinics of North America.
2007;
40
(2)
:
283-90
.
View Article PubMed Google Scholar -
Alam
M.,
Tung
R.,
Injection technique in neurotoxins and fillers: Indications, products, and outcomes. Journal of the American Academy of Dermatology.
2018;
79
(3)
:
423-35
.
View Article PubMed Google Scholar -
Nawrocki
S.,
Cha
J.,
Botulinum toxin: pharmacology and injectable administration for the treatment of primary hyperhidrosis. Journal of the American Academy of Dermatology.
2020;
82
(4)
:
969-79
.
View Article PubMed Google Scholar -
Kontochristopoulos
G.,
Platsidaki
E.,
Chemical peels in active acne and acne scars. Clinics in Dermatology.
2017;
35
(2)
:
179-82
.
View Article PubMed Google Scholar -
Ross
E.V.,
Laser Rejuvenation of Nonfacial Skin: A Review and a Personal Approach. Dermatologic Surgery.
2020;
46
:
71-6
.
View Article PubMed Google Scholar -
Taub
A.F.,
Pham
K.,
Stem Cells in Dermatology and Anti-aging Care of the Skin. Facial Plastic Surgery Clinics of North America.
2018;
26
(4)
:
425-37
.
View Article PubMed Google Scholar -
Shimizu
Y.,
Ntege
E.H.,
Sunami
H.,
Current regenerative medicine-based approaches for skin regeneration: A review of literature and a report on clinical applications in Japan. Regenerative Therapy.
2022;
21
:
73-80
.
View Article PubMed Google Scholar -
Wu
J.Y.,
Wu
S.N.,
Zhang
L.P.,
Zhao
X.S.,
Li
Y.,
Yang
Q.Y.,
Stem Cell-Derived Exosomes: A New Method for Reversing Skin Aging. Tissue Engineering and Regenerative Medicine.
2022;
19
(5)
:
961-8
.
View Article PubMed Google Scholar -
Vlassov
A.V.,
Magdaleno
S.,
Setterquist
R.,
Conrad
R.,
Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica et Biophysica Acta.
2012;
1820
(7)
:
940-8
.
View Article PubMed Google Scholar -
Narang
P.,
Shah
M.,
Beljanski
V.,
Exosomal RNAs in diagnosis and therapies. Non-Coding RNA Research.
2022;
7
(1)
:
7-15
.
View Article PubMed Google Scholar -
Kern
J.A.,
Kollipara
R.,
Hoss
E.,
Boen
M.,
Wu
D.C.,
Groff
W.,
Serious Adverse Events With Injectable Fillers: Retrospective Analysis of 7,659 Patient Outcomes. Dermatologic Surgery.
2022;
48
(5)
:
551-5
.
View Article PubMed Google Scholar -
Fitzgerald
R.,
Bertucci
V.,
Sykes
J.M.,
Duplechain
J.K.,
Adverse Reactions to Injectable Fillers. Facial Plastic Surgery.
2016;
32
(5)
:
532-55
.
View Article PubMed Google Scholar -
Cha
H.,
Hong
S.,
Park
J.H.,
Park
H.H.,
Stem Cell-Derived Exosomes and Nanovesicles: Promotion of Cell Proliferation, Migration, and Anti-Senescence for Treatment of Wound Damage and Skin Ageing. Pharmaceutics.
2020;
12
(12)
:
1135
.
View Article PubMed Google Scholar -
Cai
J.,
Xu
J.,
Ye
Z.,
Wang
L.,
Zheng
T.,
Zhang
T.,
Exosomes Derived From Kartogenin-Preconditioned Mesenchymal Stem Cells Promote Cartilage Formation and Collagen Maturation for Enthesis Regeneration in a Rat Model of Chronic Rotator Cuff Tear. The American Journal of Sports Medicine.
2023;
51
(5)
:
1267-76
.
View Article PubMed Google Scholar -
Zhang
Y.,
Liu
Y.,
Liu
H.,
Tang
W.H.,
Exosomes: biogenesis, biologic function and clinical potential. Cell & Bioscience.
2019;
9
(1)
:
19
.
View Article PubMed Google Scholar -
Hu
Y.,
Wang
Y.,
Chen
T.,
Hao
Z.,
Cai
L.,
Li
J.,
Exosome: Function and Application in Inflammatory Bone Diseases. Oxidative Medicine and Cellular Longevity.
2021;
2021
(1)
:
6324912
.
View Article PubMed Google Scholar -
Wang
S.,
Lei
B.,
Zhang
E.,
Gong
P.,
Gu
J.,
He
L.,
Targeted Therapy for Inflammatory Diseases with Mesenchymal Stem Cells and Their Derived Exosomes: From Basic to Clinics. International Journal of Nanomedicine.
2022;
17
:
1757-81
.
View Article PubMed Google Scholar -
Ren
K.,
Exosomes in perspective: a potential surrogate for stem cell therapy. Odontology.
2019;
107
(3)
:
271-84
.
View Article PubMed Google Scholar -
Basu
J.,
Ludlow
J.W.,
Exosomes for repair, regeneration and rejuvenation. Expert Opinion on Biological Therapy.
2016;
16
(4)
:
489-506
.
View Article PubMed Google Scholar -
Fadilah
N.I. Md,
Jailani
M.S. Mohd Abdul Kader,
Hisham
M.A. Badrul,
Raj
N. Sunthar,
Shamsuddin
S.A.,
Ng
M.H.,
Cell secretomes for wound healing and tissue regeneration: next generation acellular based tissue engineered products. Journal of Tissue Engineering.
2022;
13
:
20417314221114273
.
View Article PubMed Google Scholar -
Al-Masawa
M.E.,
Alshawsh
M.A.,
Ng
C.Y.,
Ng
A.M.,
Foo
J.B.,
Vijakumaran
U.,
Efficacy and safety of small extracellular vesicle interventions in wound healing and skin regeneration: A systematic review and meta-analysis of animal studies. Theranostics.
2022;
12
(15)
:
6455-508
.
View Article PubMed Google Scholar -
Jella
K.K.,
Nasti
T.H.,
Li
Z.,
Malla
S.R.,
Buchwald
Z.S.,
Khan
M.K.,
Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines.
2018;
6
(4)
:
69
.
View Article PubMed Google Scholar -
Pathania
A.S.,
Challagundla
K.B.,
Exosomal Long Non-coding RNAs: Emerging Players in the Tumor Microenvironment. Molecular Therapy. Nucleic Acids.
2020;
23
:
1371-83
.
View Article PubMed Google Scholar -
Li
W.,
Wang
X.,
Li
C.,
Chen
T.,
Yang
Q.,
Exosomal non-coding RNAs: emerging roles in bilateral communication between cancer cells and macrophages. Molecular Therapy.
2022;
30
(3)
:
1036-53
.
View Article PubMed Google Scholar -
Zou
J.,
Peng
H.,
Liu
Y.,
The Roles of Exosomes in Immunoregulation and Autoimmune Thyroid Diseases. Frontiers in Immunology.
2021;
12
:
757674
.
View Article PubMed Google Scholar -
Greening
D.W.,
Gopal
S.K.,
Xu
R.,
Simpson
R.J.,
Chen
W.,
Exosomes and their roles in immune regulation and cancer. Seminars in Cell & Developmental Biology.
2015;
40
:
72-81
.
View Article PubMed Google Scholar -
Dai
J.,
Su
Y.,
Zhong
S.,
Cong
L.,
Liu
B.,
Yang
J.,
Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduction and Targeted Therapy.
2020;
5
(1)
:
145
.
View Article PubMed Google Scholar -
Xu
X.,
Xu
L.,
Wen
C.,
Xia
J.,
Zhang
Y.,
Liang
Y.,
Programming assembly of biomimetic exosomes: an emerging theranostic nanomedicine platform. Materials Today. Bio.
2023;
22
:
100760
.
View Article PubMed Google Scholar -
Suzuki
E.,
Fujita
D.,
Takahashi
M.,
Oba
S.,
Nishimatsu
H.,
Stem cell-derived exosomes as a therapeutic tool for cardiovascular disease. World Journal of Stem Cells.
2016;
8
(9)
:
297-305
.
View Article PubMed Google Scholar -
Norozi
F.,
Ahmadzadeh
A.,
Shahrabi
S.,
Vosoughi
T.,
Saki
N.,
Mesenchymal stem cells as a double-edged sword in suppression or progression of solid tumor cells. Tumour Biology.
2016;
37
(9)
:
11679-89
.
View Article PubMed Google Scholar -
Li
Y.H.,
Feng
L.,
Zhang
G.X.,
Ma
C.G.,
Intranasal delivery of stem cells as therapy for central nervous system disease. Experimental and Molecular Pathology.
2015;
98
(2)
:
145-51
.
View Article PubMed Google Scholar -
van Velthoven
C.T.,
Kavelaars
A.,
van Bel
F.,
Heijnen
C.J.,
Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatric Research.
2010;
68
(5)
:
419-22
.
View Article PubMed Google Scholar -
Jiang
Y.,
Zhu
J.,
Xu
G.,
Liu
X.,
Intranasal delivery of stem cells to the brain. Expert Opinion on Drug Delivery.
2011;
8
(5)
:
623-32
.
View Article PubMed Google Scholar -
Ridder
K.,
Sevko
A.,
Heide
J.,
Dams
M.,
Rupp
A.K.,
Macas
J.,
Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. OncoImmunology.
2015;
4
(6)
:
e1008371
.
View Article PubMed Google Scholar -
He
J.,
Ren
W.,
Wang
W.,
Han
W.,
Jiang
L.,
Zhang
D.,
Exosomal targeting and its potential clinical application. Drug Delivery and Translational Research.
2022;
12
(10)
:
2385-402
.
View Article PubMed Google Scholar -
Yin
W.,
Ma
H.,
Qu
Y.,
Wang
S.,
Zhao
R.,
Yang
Y.,
Targeted exosome-based nanoplatform for new-generation therapeutic strategies. Biomedical Materials (Bristol, England).
2024;
19
(3)
:
032002
.
View Article PubMed Google Scholar -
Lin
Y.,
Lu
Y.,
Li
X.,
Biological characteristics of exosomes and genetically engineered exosomes for the targeted delivery of therapeutic agents. Journal of Drug Targeting.
2020;
28
(2)
:
129-41
.
View Article PubMed Google Scholar -
Sen
S.,
Current Strategies for Promoting the Large-scale Production of Exosomes. Current Neuropharmacology.
2023;
21
(9)
:
1964
.
-
Choi
H.,
Choi
Y.,
Yim
H.Y.,
Mirzaaghasi
A.,
Yoo
J.K.,
Choi
C.,
Biodistribution of Exosomes and Engineering Strategies for Targeted Delivery of Therapeutic Exosomes. Tissue Engineering and Regenerative Medicine.
2021;
18
(4)
:
499-511
.
View Article PubMed Google Scholar -
Qu
Q.,
Fu
B.,
Long
Y.,
Liu
Z.Y.,
Tian
X.H.,
Current Strategies for Promoting the Large-scale Production of Exosomes. Current Neuropharmacology.
2023;
21
(9)
:
1964-79
.
View Article PubMed Google Scholar -
Wu
G.,
Zhang
J.,
Zhao
Q.,
Zhuang
W.,
Ding
J.,
Zhang
C.,
Molecularly Engineered Macrophage-Derived Exosomes with Inflammation Tropism and Intrinsic Heme Biosynthesis for Atherosclerosis Treatment. Angewandte Chemie International Edition in English.
2020;
59
(10)
:
4068-74
.
View Article PubMed Google Scholar -
Abdelsalam
M.,
Ahmed
M.,
Osaid
Z.,
Hamoudi
R.,
Harati
R.,
Insights into Exosome Transport through the Blood-Brain Barrier and the Potential Therapeutical Applications in Brain Diseases. Pharmaceuticals (Basel, Switzerland).
2023;
16
(4)
:
571
.
View Article PubMed Google Scholar -
Ahn
S.H.,
Ryu
S.W.,
Choi
H.,
You
S.,
Park
J.,
Choi
C.,
Manufacturing Therapeutic Exosomes: from Bench to Industry. Molecules and Cells.
2022;
45
(5)
:
284-90
.
View Article PubMed Google Scholar -
Wang
C.K.,
Tsai
T.H.,
Lee
C.H.,
Regulation of exosomes as biologic medicines: regulatory challenges faced in exosome development and manufacturing processes. Clinical and Translational Science.
2024;
17
(8)
:
e13904
.
View Article PubMed Google Scholar -
Zhang
Q.,
Fu
L.,
Liang
Y.,
Guo
Z.,
Wang
L.,
Ma
C.,
Exosomes originating from MSCs stimulated with TGF-β and IFN-γ promote Treg differentiation. Journal of Cellular Physiology.
2018;
233
(9)
:
6832-40
.
View Article PubMed Google Scholar -
Haraszti
R.A.,
Miller
R.,
Stoppato
M.,
Sere
Y.Y.,
Coles
A.,
Didiot
M.C.,
Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Molecular Therapy.
2018;
26
(12)
:
2838-47
.
View Article PubMed Google Scholar -
Alshahrani
M.Y.,
Jasim
S.A.,
Altalbawy
F.M.,
Bansal
P.,
Kaur
H.,
Al-Hamdani
M.M.,
A comprehensive insight into the immunomodulatory role of MSCs-derived exosomes (MSC-Exos) through modulating pattern-recognition receptors (PRRs). Cell Biochemistry and Function.
2024;
42
(4)
:
e4029
.
View Article PubMed Google Scholar -
Teo
K.Y.,
Zhang
S.,
Loh
J.T.,
Lai
R.C.,
Hey
H.W.,
Lam
K.P.,
Mesenchymal Stromal Cell Exosomes Mediate M2-like Macrophage Polarization through CD73/Ecto-5'-Nucleotidase Activity. Pharmaceutics.
2023;
15
(5)
:
1489
.
View Article PubMed Google Scholar -
Su
W.,
Yin
Y.,
Zhao
J.,
Hu
R.,
Zhang
H.,
Hu
J.,
Exosomes derived from umbilical cord-derived mesenchymal stem cells exposed to diabetic microenvironment enhance M2 macrophage polarization and protect against diabetic nephropathy. The FASEB Journal.
2024;
38
(14)
:
e23798
.
View Article PubMed Google Scholar -
Zheng
T.,
Li
S.,
Zhang
T.,
Fu
W.,
Liu
S.,
He
Y.,
Exosome-shuttled miR-150-5p from LPS-preconditioned mesenchymal stem cells down-regulate PI3K/Akt/mTOR pathway via Irs1 to enhance M2 macrophage polarization and confer protection against sepsis. Frontiers in Immunology.
2024;
15
:
1397722
.
View Article PubMed Google Scholar -
Tian
H.,
Chen
A.,
Gao
P.,
Wang
F.,
Zhao
Y.,
Wang
F.,
Human Umbilical Cord Mesenchymal Stem Cell-derived Exosomes Induce Macrophage M2 Polarization by Antagonizing LPS-mediated Stimulation of the NF-κB and STAT3 Pathways. Combinatorial Chemistry & High Throughput Screening.
2024;
27
.
View Article PubMed Google Scholar -
Soufihasanabad
S.,
Mahmoudi
M.,
Taghavi-Farahabadi
M.,
Mirsanei
Z.,
Mahmoudi Lamouki
R.,
Mirza Abdalla
J.K.,
In vivo polarization of M2 macrophages by mesenchymal stem cell-derived extracellular vesicles: A novel approach to macrophage polarization and its potential in treating inflammatory diseases. Medical Hypotheses.
2024;
187
.
View Article Google Scholar -
Ma
D.,
Wu
Z.,
Zhao
X.,
Zhu
X.,
An
Q.,
Wang
Y.,
Immunomodulatory effects of umbilical mesenchymal stem cell-derived exosomes on CD4+ T cells in patients with primary Sjögren's syndrome. Inflammopharmacology.
2023;
31
(4)
:
1823-38
.
View Article PubMed Google Scholar -
Franco da Cunha
F.,
Andrade-Oliveira
V.,
Candido de Almeida
D.,
Borges da Silva
T.,
Naffah de Souza Breda
C.,
Costa Cruz
M.,
Extracellular Vesicles isolated from Mesenchymal Stromal Cells Modulate CD4+ T Lymphocytes Toward a Regulatory Profile. Cells.
2020;
9
(4)
:
1059
.
View Article PubMed Google Scholar -
Lee
S.,
Kim
S.,
Chung
H.,
Moon
J.H.,
Kang
S.J.,
Park
C.G.,
Mesenchymal stem cell-derived exosomes suppress proliferation of T cells by inducing cell cycle arrest through p27kip1/Cdk2 signaling. Immunology Letters.
2020;
225
:
16-22
.
View Article PubMed Google Scholar -
Duan
Y.,
Chen
X.,
Shao
H.,
Li
Y.,
Zhang
Z.,
Li
H.,
Enhanced immunosuppressive capability of mesenchymal stem cell-derived small extracellular vesicles with high expression of CD73 in experimental autoimmune uveitis. Stem Cell Research {&}amp; Therapy.
2024;
15
(1)
:
149
.
View Article PubMed Google Scholar -
Feng
Y.,
Bao
X.,
Zhao
J.,
Kang
L.,
Sun
X.,
Xu
B.,
MSC-Derived Exosomes Mitigate Myocardial Ischemia/Reperfusion Injury by Reducing Neutrophil Infiltration and the Formation of Neutrophil Extracellular Traps. International Journal of Nanomedicine.
2024;
19
:
2071-90
.
View Article PubMed Google Scholar -
Frantz
C.,
Stewart
K.M.,
Weaver
V.M.,
The extracellular matrix at a glance. Journal of Cell Science.
2010;
123
(Pt 24)
:
4195-200
.
View Article PubMed Google Scholar -
Quan
T.,
Fisher
G.J.,
Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology.
2015;
61
(5)
:
427-34
.
View Article PubMed Google Scholar -
Yasui
T.,
Yonetsu
M.,
Tanaka
R.,
Tanaka
Y.,
Fukushima
S.,
Yamashita
T.,
In vivo observation of age-related structural changes of dermal collagen in human facial skin using collagen-sensitive second harmonic generation microscope equipped with 1250-nm mode-locked Cr:Forsterite laser. Journal of Biomedical Optics.
2013;
18
(3)
:
31108
.
View Article PubMed Google Scholar -
Katz
B.E.,
Lewis
J.,
McHugh
L.,
Pellegrino
A.,
Popescu
L.,
The Tolerability and Efficacy of a Three-product Anti-aging Treatment Regimen in Subjects with Moderate-to-severe Photodamage. The Journal of Clinical and Aesthetic Dermatology.
2015;
8
(10)
:
21-6
.
PubMed Google Scholar -
Kockaert
M.,
Neumann
M.,
Systemic and topical drugs for aging skin. Journal of Drugs in Dermatology.
2003;
2
(4)
:
435-41
.
PubMed Google Scholar -
Geesin
J.C.,
Gordon
J.S.,
Berg
R.A.,
Retinoids affect collagen synthesis through inhibition of ascorbate-induced lipid peroxidation in cultured human dermal fibroblasts. Archives of Biochemistry and Biophysics.
1990;
278
(2)
:
350-5
.
View Article PubMed Google Scholar -
Alster
T.S.,
Graham
P.M.,
Microneedling: A Review and Practical Guide. Dermatologic Surgery.
2018;
44
(3)
:
397-404
.
View Article PubMed Google Scholar -
Badran
K.W.,
Nabili
V.,
Lasers, Microneedling, and Platelet-Rich Plasma for Skin Rejuvenation and Repair. Facial Plastic Surgery Clinics of North America.
2018;
26
(4)
:
455-68
.
View Article PubMed Google Scholar -
Juhasz
M.L.,
Cohen
J.L.,
Microneedling for the Treatment of Scars: An Update for Clinicians. Clinical, Cosmetic and Investigational Dermatology.
2020;
13
:
997-1003
.
View Article PubMed Google Scholar -
Farrukh
A.K.,
Ahmad
S.,
Mehrose
M.Y.,
Saleem
M.,
Yousaf
M.A.,
Mujahid
A.M.,
Efficacy Of Micro-Needling On Post Acne Scars. Journal of Ayub Medical College, Abbottabad.
2019;
31
(3)
:
336-9
.
PubMed Google Scholar -
Huang
J.,
Xiong
J.,
Yang
L.,
Zhang
J.,
Sun
S.,
Liang
Y.,
Cell-free exosome-laden scaffolds for tissue repair. Nanoscale.
2021;
13
(19)
:
8740-50
.
View Article PubMed Google Scholar -
Ni
Z.,
Zhou
S.,
Li
S.,
Kuang
L.,
Chen
H.,
Luo
X.,
Exosomes: roles and therapeutic potential in osteoarthritis. Bone Research.
2020;
8
(1)
:
25
.
View Article PubMed Google Scholar -
Jing
H.,
He
X.,
Zheng
J.,
Exosomes and regenerative medicine: state of the art and perspectives. Translational Research ; the Journal of Laboratory and Clinical Medicine.
2018;
196
:
1-16
.
View Article PubMed Google Scholar -
Jablonska-Trypuc
A.,
Matejczyk
M.,
Rosochacki
S.,
Enhanced Type I Collagen Synthesis in Fibroblasts by Dermal Stem/Progenitor Cell-Derived Exosomes. Biological and Pharmaceutical Bulletin.
2022;
45
(7)
:
872-80
.
View Article Google Scholar -
Yang
G.H.,
Lee
Y.B.,
Kang
D.,
Choi
E.,
Nam
Y.,
Lee
K.H.,
Overcome the barriers of the skin: exosome therapy. Biomaterials Research.
2021;
25
(1)
:
22
.
View Article PubMed Google Scholar -
Zhao
H.,
Li
Z.,
Wang
Y.,
Zhou
K.,
Li
H.,
Bi
S.,
Bioengineered MSC-derived exosomes in skin wound repair and regeneration. Frontiers in Cell and Developmental Biology.
2023;
11
:
1029671
.
View Article PubMed Google Scholar -
Moghadasi
S.,
Elveny
M.,
Rahman
H.S.,
Suksatan
W.,
Jalil
A.T.,
Abdelbasset
W.K.,
A paradigm shift in cell-free approach: the emerging role of MSCs-derived exosomes in regenerative medicine. Journal of Translational Medicine.
2021;
19
(1)
:
302
.
View Article PubMed Google Scholar -
Nikfarjam
S.,
Rezaie
J.,
Zolbanin
N.M.,
Jafari
R.,
Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. Journal of Translational Medicine.
2020;
18
(1)
:
449
.
View Article PubMed Google Scholar -
Li
J.,
Li
Z.,
Wang
S.,
Bi
J.,
Huo
R.,
Exosomes from human adipose-derived mesenchymal stem cells inhibit production of extracellular matrix in keloid fibroblasts via downregulating transforming growth factor-β2 and Notch-1 expression. Bioengineered.
2022;
13
(4)
:
8515-25
.
View Article PubMed Google Scholar -
Zhang
P.,
Wu
P.,
Khan
U.Z.,
Zhou
Z.,
Sui
X.,
Li
C.,
Exosomes derived from LPS-preconditioned bone marrow-derived MSC modulate macrophage plasticity to promote allograft survival via the NF-κB/NLRP3 signaling pathway. Journal of Nanobiotechnology.
2023;
21
(1)
:
332
.
View Article PubMed Google Scholar -
Liu
X.,
Wang
S.,
Wu
S.,
Hao
Q.,
Li
Y.,
Guo
Z.,
Exosomes secreted by adipose-derived mesenchymal stem cells regulate type I collagen metabolism in fibroblasts from women with stress urinary incontinence. Stem Cell Research & Therapy.
2018;
9
(1)
:
159
.
View Article PubMed Google Scholar -
Samiei
M.,
Alipour
M.,
Khezri
K.,
Saadat
Y.R.,
Forouhandeh
H.,
Abdolahinia
E.D.,
Application of Collagen and Mesenchymal Stem Cells in Regenerative Dentistry. Current Stem Cell Research & Therapy.
2022;
17
(7)
:
606-20
.
View Article PubMed Google Scholar -
Trentini
M.,
Zanolla
I.,
Zanotti
F.,
Tiengo
E.,
Licastro
D.,
Dal Monego
S.,
Apple Derived Exosomes Improve Collagen Type I Production and Decrease MMPs during Aging of the Skin through Downregulation of the NF-κB Pathway as Mode of Action. Cells.
2022;
11
(24)
:
3950
.
View Article PubMed Google Scholar -
Wang
T.,
Jian
Z.,
Baskys
A.,
Yang
J.,
Li
J.,
Guo
H.,
MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials.
2020;
257
:
120264
.
View Article PubMed Google Scholar -
Fisher
G.J.,
Quan
T.,
Purohit
T.,
Shao
Y.,
Cho
M.K.,
He
T.,
Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. American Journal of Pathology.
2009;
174
(1)
:
101-14
.
View Article PubMed Google Scholar -
Liu
J.,
Li
F.,
Liu
B.,
Yao
Z.,
Li
L.,
Liu
G.,
Adipose-derived mesenchymal stem cell exosomes inhibit transforming growth factor-β1-induced collagen synthesis in oral mucosal fibroblasts. Experimental and Therapeutic Medicine.
2021;
22
(6)
:
1419
.
View Article PubMed Google Scholar -
Yu
F.,
J. Chen,
X. Wang,
Q. Cai,
K. Chen,
Y. He,
MSC-derived exosomes prevent peritoneal fibroblast transdifferentiation in peritoneal dialysis-associated peritoneal fibrosis. Nephrology Dialysis Transplantation.
2023;
38
(Supplement_1)
:
gfad063c_4662
.
View Article Google Scholar -
Li
F.Q.,
Chen
W.B.,
Luo
Z.W.,
Chen
Y.S.,
Sun
Y.Y.,
Su
X.P.,
Bone marrow mesenchymal stem cell-derived exosomal microRNAs target PI3K/Akt signaling pathway to promote the activation of fibroblasts. World Journal of Stem Cells.
2023;
15
(4)
:
248-67
.
View Article PubMed Google Scholar -
Zhao
W.,
Zhang
R.,
Zang
C.,
Zhang
L.,
Zhao
R.,
Li
Q.,
Exosome Derived from Mesenchymal Stem Cells Alleviates Pathological Scars by Inhibiting the Proliferation, Migration and Protein Expression of Fibroblasts via Delivering miR-138-5p to Target SIRT1. International Journal of Nanomedicine.
2022;
17
:
4023-38
.
View Article PubMed Google Scholar -
Hu
L.,
Wang
J.,
Zhou
X.,
Xiong
Z.,
Zhao
J.,
Yu
R.,
Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Scientific Reports.
2016;
6
(1)
:
32993
.
View Article PubMed Google Scholar -
Wang
L.,
Hu
L.,
Zhou
X.,
Xiong
Z.,
Zhang
C.,
Shehada
H.M.,
Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Scientific Reports.
2017;
7
(1)
:
13321
.
View Article PubMed Google Scholar -
Zhang
Y.,
Pan
Y.,
Liu
Y.,
Li
X.,
Tang
L.,
Duan
M.,
Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Research & Therapy.
2021;
12
(1)
:
434
.
View Article PubMed Google Scholar -
Xu
X.,
Wang
B.,
Ren
C.,
Hu
J.,
Greenberg
D.A.,
Chen
T.,
Age-related Impairment of Vascular Structure and Functions. Aging and Disease.
2017;
8
(5)
:
590-610
.
View Article PubMed Google Scholar -
Kataru
R.P.,
Park
H.J.,
Shin
J.,
Baik
J.E.,
Sarker
A.,
Brown
S.,
Structural and Functional Changes in Aged Skin Lymphatic Vessels. Frontiers in Aging.
2022;
3
:
864860
.
View Article PubMed Google Scholar -
Ivanna
A.-S.,
Age features of functional condition of microvessel endothelia. Ageing and longevity.
2022;
3
(1)
:
8-13
.
-
Holowatz
L.A.,
Human cutaneous microvascular ageing: potential insights into underlying physiological mechanisms of endothelial function and dysfunction. The Journal of Physiology.
2008;
586
(14)
:
3301
.
View Article PubMed Google Scholar -
Katusic
Z.S.,
Mechanisms of endothelial dysfunction induced by aging: role of arginase I. Circulation Research.
2007;
101
(7)
:
640-1
.
View Article PubMed Google Scholar -
Ungvari
Z.,
Tarantini
S.,
Kiss
T.,
Wren
J.D.,
Giles
C.B.,
Griffin
C.T.,
Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nature Reviews. Cardiology.
2018;
15
(9)
:
555-65
.
View Article PubMed Google Scholar -
Ribeiro
M.F.,
Zhu
H.,
Millard
R.W.,
Fan
G.C.,
Exosomes Function in Pro- and Anti-Angiogenesis. Current Angiogenesis.
2013;
2
(1)
:
54-9
.
View Article PubMed Google Scholar -
Liang
X.,
Zhang
L.,
Wang
S.,
Han
Q.,
Zhao
R.C.,
Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. Journal of Cell Science.
2016;
129
(11)
:
2182-9
.
View Article PubMed Google Scholar -
Bian
D.,
Wu
Y.,
Song
G.,
Azizi
R.,
Zamani
A.,
The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Research & Therapy.
2022;
13
(1)
:
24
.
View Article PubMed Google Scholar -
Teng
L.,
Maqsood
M.,
Zhu
M.,
Zhou
Y.,
Kang
M.,
Zhou
J.,
Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Diabetic Wound Healing via Promoting M2 Macrophage Polarization, Angiogenesis, and Collagen Deposition. International Journal of Molecular Sciences.
2022;
23
(18)
:
10421
.
View Article PubMed Google Scholar -
Vu
N.B.,
Nguyen
H.T.,
Palumbo
R.,
Pellicano
R.,
Fagoonee
S.,
Pham
P.V.,
Stem cell-derived exosomes for wound healing: current status and promising directions. Minerva Medica.
2021;
112
(3)
:
384-400
.
View Article PubMed Google Scholar -
Zhang
L.,
Ouyang
P.,
He
G.,
Wang
X.,
Song
D.,
Yang
Y.,
Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. Journal of Cellular and Molecular Medicine.
2021;
25
(4)
:
2148-62
.
View Article PubMed Google Scholar -
Pan
Q.,
Wang
Y.,
Lan
Q.,
Wu
W.,
Li
Z.,
Ma
X.,
Exosomes Derived from Mesenchymal Stem Cells Ameliorate Hypoxia/Reoxygenation-Injured ECs via Transferring MicroRNA-126. Stem Cells International.
2019;
2019
(1)
:
2831756
.
View Article PubMed Google Scholar -
Nie
W.,
Huang
X.,
Zhao
L.,
Wang
T.,
Zhang
D.,
Xu
T.,
Exosomal miR-17-92 derived from human mesenchymal stem cells promotes wound healing by enhancing angiogenesis and inhibiting endothelial cell ferroptosis. Tissue & Cell.
2023;
83
.
View Article PubMed Google Scholar -
Zheng
L.,
Song
H.,
Li
Y.,
Li
H.,
Lin
G.,
Cai
Z.,
Insulin-Induced Gene 1-Enhance Secretion of BMSC Exosome Enriched in miR-132-3p Promoting Wound Healing in Diabetic Mice. Molecular Pharmaceutics.
2024;
21
(9)
:
4372-85
.
View Article PubMed Google Scholar -
Gonzalez-King
H.,
García
N.A.,
Ontoria-Oviedo
I.,
Ciria
M.,
Montero
J.A.,
Sepúlveda
P.,
Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells (Dayton, Ohio).
2017;
35
(7)
:
1747-59
.
View Article PubMed Google Scholar -
Lee
Y.I.,
Choi
S.,
Roh
W.S.,
Lee
J.H.,
Kim
T.G.,
Cellular Senescence and Inflammaging in the Skin Microenvironment. International Journal of Molecular Sciences.
2021;
22
(8)
:
3849
.
View Article PubMed Google Scholar -
Li
X.,
Li
C.,
Zhang
W.,
Wang
Y.,
Qian
P.,
Huang
H.,
Inflammation and aging: signaling pathways and intervention therapies. Signal Transduction and Targeted Therapy.
2023;
8
(1)
:
239
.
View Article PubMed Google Scholar -
Jarrold
B.B.,
Inflammaging in human photoexposed skin: Early onset of senescence and imbalanced epidermal homeostasis across the decades. bioRxiv.
2022;
:
2022.03.28.486066
.
-
Radhiga
T.,
Phytochemicals as modulators of ultraviolet-b radiation induced cellular and molecular events: A review. Journal of Radiation and Cancer Research.
2016;
7
(1)
:
2-12
.
View Article Google Scholar -
Mukherjee
A.,
Das
B.,
The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomaterials and Biosystems.
2024;
13
:
100090
.
View Article PubMed Google Scholar -
Mirastschijski
U.,
Lupše
B.,
Maedler
K.,
Sarma
B.,
Radtke
A.,
Belge
G.,
Matrix Metalloproteinase-3 is Key Effector of TNF-α-Induced Collagen Degradation in Skin. International Journal of Molecular Sciences.
2019;
20
(20)
:
5234
.
View Article PubMed Google Scholar -
Draelos
Z.D.,
Sugar Sag: What Is Skin Glycation and How Do You Combat It?. Journal of Drugs in Dermatology.
2024;
23
(4)
:
SF378083s5-SF378083s10
.
-
Sultana
R.,
Parveen
A.,
Kang
M.C.,
Hong
S.M.,
Kim
S.Y.,
Glyoxal-derived advanced glycation end products (GO-AGEs) with UVB critically induce skin inflammaging: in vitro and in silico approaches. Scientific Reports.
2024;
14
(1)
:
1843
.
View Article PubMed Google Scholar -
Zheng
W.,
Li
H.,
Go
Y.,
Chan
X.H.,
Huang
Q.,
Wu
J.,
Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors. Nutrients.
2022;
14
(21)
:
4588
.
View Article PubMed Google Scholar -
Bahadorani
M.,
Nasiri
M.,
Dellinger
K.,
Aravamudhan
S.,
Zadegan
R.,
Engineering Exosomes for Therapeutic Applications: Decoding Biogenesis, Content Modification, and Cargo Loading Strategies. International Journal of Nanomedicine.
2024;
19
:
7137-64
.
View Article PubMed Google Scholar -
Sadeghi
M.,
Mohammadi
M.,
Afshari
J. Tavakol,
Iranparast
S.,
Ansari
B.,
Dehnavi
S.,
Therapeutic potential of mesenchymal stem cell-derived exosomes for allergic airway inflammation. Cellular Immunology.
2024;
:
104813
.
View Article PubMed Google Scholar -
Liu
X.,
Wei
Q.,
Lu
L.,
Cui
S.,
Ma
K.,
Zhang
W.,
Immunomodulatory potential of mesenchymal stem cell-derived extracellular vesicles: targeting immune cells. Frontiers in Immunology.
2023;
14
:
1094685
.
View Article PubMed Google Scholar -
Lin
T.Y.,
Chang
T.M.,
Tsai
W.C.,
Hsieh
Y.J.,
Wang
L.T.,
Huang
H.C.,
Human Umbilical Cord Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Reduce Skin Inflammation In Vitro. International Journal of Molecular Sciences.
2023;
24
(23)
:
17109
.
View Article PubMed Google Scholar -
Cho
B.,
1615 Adipose stem cell exosome (ASCE): next generation regenerative aesthetics & therapeutics for scalp & hair. The Journal of Investigative Dermatology.
2023;
143
(5)
:
277
.
View Article Google Scholar -
Cho
B.S.,
Kim
J.O.,
Ha
D.H.,
Yi
Y.W.,
Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Research & Therapy.
2018;
9
(1)
:
187
.
View Article PubMed Google Scholar -
Gorgun
C.,
Preconditioned Mesenchymal Stromal Cell-Derived Extracellular Vesicles (EVs) Counteract Inflammaging. Cells.
2022;
11
(22)
:
3695
.
View Article Google Scholar -
Zhang
Y.,
Yan
J.,
Li
Z.,
Zheng
J.,
Sun
Q.,
Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Psoriasis-like Skin Inflammation. Journal of Interferon & Cytokine Research.
2022;
42
(1)
:
8-18
.
View Article PubMed Google Scholar
Comments
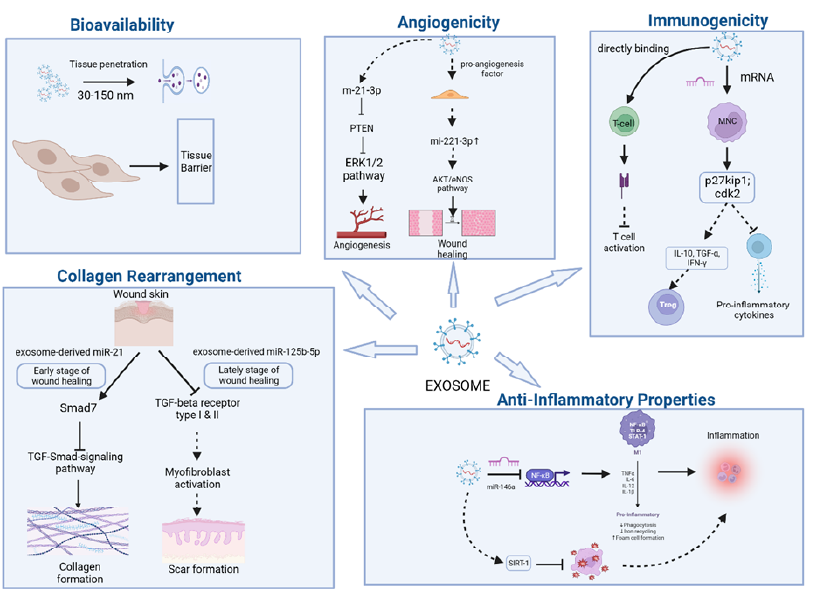
Article Details
Volume & Issue : Vol 11 No 12 (2024)
Page No.: 7003-7014
Published on: 2024-12-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 2530 times
- PDF downloaded - 525 times
- XML downloaded - 74 times
 Biomedpress
Biomedpress



