Abstract
Stroke is a debilitating neurological disorder that frequently results in cognitive impairments, significantly affecting the quality of life of post-stroke patients. Current therapeutic options for poststroke cognitive impairment (PSCI) are limited. Mesenchymal stem cells (MSCs) have emerged as a promising strategy for enhancing neurological recovery, including cognitive function. This review evaluates the application of MSCs in improving cognitive function in stroke patients, focusing on data from preclinical studies. Approximately 75% of strokes occur in the elderly population, and animal models have been developed to study the effects of MSCs on ischemic stroke in aged rats, as well as in the presence of comorbidities such as hypertension and diabetes. The potential of MSCs to enhance cognitive function following a stroke is believed to involve multiple mechanisms, including the secretion of trophic factors, immunomodulation, differentiation into neural cell types, promotion of angiogenesis, and replacement of damaged cells. However, several challenges remain to be addressed, including a lack of clear understanding of the biological processes contributing to MSC efficacy, standardization of MSC preparation, and determination of optimal treatment protocols. Further studies on the specific mechanisms of action and clinical application of MSCs are required to confirm their therapeutic efficacy for stroke patients. The development of innovative and targeted therapies for PSCI will ultimately improve the quality of life for stroke survivors.
Introduction
Stroke is a neurological deficit attributed to a sudden disruption in cerebral blood flow. There are two types of strokes: ischemic strokes, which comprise 87% of the cases and are caused by blockages in blood flow, and hemorrhagic strokes, which account for 13% and are caused by vessel rupture1. Annually, stroke affects approximately 15 million people globally, with five million fatalities and another five million people experiencing long-term disabilities2. At present, treatment options for stroke patients are restricted to dissolving thrombi using tissue plasminogen activators like alteplase or performing mechanical thrombectomy3. Thrombolysis is highly effective in treating strokes within 4.5 hours of onset, but its efficacy is limited for large thrombi or severe strokes4. Unfortunately, fewer than 5% of patients with ischemic stroke have access to these treatments, and many continue to experience neurological deficits post-treatment, with no available therapy to aid recovery5. Consequently, stroke patients may experience cognitive deficits in memory, language, attention, executive function, and orientation domains, significantly affecting their functional outcomes6.
Recently, the application of mesenchymal stem cells (MSCs) has gained significant attention as a promising therapy for stroke. Mesenchymal stem cells exert therapeutic effects through their capacity to proliferate, differentiate into various cell types, and regulate immune responses7. Mesenchymal stem cells have anti-inflammatory, anti-apoptotic, angiogenic, and neurogenic effects, which may contribute to their therapeutic potentials3. While several reviews have explored MSC therapy in stroke, our study focuses on preclinical evidence specifically related to cognitive improvement. Preclinical studies play a crucial role in understanding the potential mechanisms, efficacy, and safety of MSC therapy before it can be translated into clinical applications. Unlike many existing reviews that focus broadly on stroke recovery, this paper specifically highlights the effects of MSCs on cognitive function post-stroke. Additionally, this review discusses studies using animal models with comorbidities like aging, hypertension, and diabetes, which are common in stroke patients but often overlooked in research8.
Stroke and Cognitive Impairment
Post-stroke cognitive impairment (PSCI) is common and related to poor patient outcomes. Recent studies suggest that 20-80% of stroke survivors experience cognitive deficits within the first three months post-stroke, and 7-23% of them progress to post-stroke dementia9. Post-stroke cognitive impairment affects multiple cognitive domains such as memory, attention, executive function, language, and visuospatial skills. These deficits can significantly impact the quality of life, independence, and overall functionality of patients with stroke. Individuals with PSCI may struggle with daily activities, maintain social relationships, and return to work, leading to increased disability and reliance on others10. Additionally, PSCI is associated with a higher risk of developing dementia, highlighting the importance of early detection and intervention9. As shown in Table 1, several neural regions affected by stroke can have a significant impact on cognitive function.
| Brain area damage | Cognitive Function Impaired | References |
| Frontal Lobe | Executive function, attention, working memory, behaviour change | 11 |
| Parietal Lobe | Visuospatial abilities, attention, language, body awareness | 12 |
| Temporal Lobe | Memory, language, auditory processing | 12 |
| Hippocampus | Episodic memory formation and retrieval | 12 |
| Amygdala | Emotional processing, fear conditioning, memory modulation | 12 |
| Basal Ganglia | Motor control, procedural learning, cognitive flexibility | 12 |
| Cerebellum | Coordination, motor skills, working memory, language processing | 12 |
Given the significant impact of PSCI on patients' quality of life and the limitations of current therapeutic approaches, there is an urgent need for innovative treatments that can effectively address cognitive deficits following stroke. Despite notable advances in stroke treatment and rehabilitation, effective therapies for PSCI remain limited. Current strategies, including pharmacological treatments and cognitive rehabilitation, offer only modest improvements in cognitive function following stroke and often come with limitations in terms of efficacy, safety, and applicability across diverse patient populations13. Pharmacological treatments, such as cholinesterase inhibitors and memantine, have produced mixed and inconclusive results regarding their effectiveness in treating PSCI, and may also have side effects or be unsuitable for some patients14. Cognitive rehabilitation has the potential to enhance specific cognitive areas, but the benefit of these improvements to everyday functioning and long-term outcomes remains uncertain, and access to specialized cognitive rehabilitation services can be limited in resource-poor settings15. The mechanisms underlying PSCI include oxidative stress, neuroinflammation, and impaired synaptic plasticity16, 17. Mesenchymal stem cells have emerged as a promising therapeutic approach to address these pathologies due to their multifaceted properties. Given the potential of mesenchymal stem cells, it is crucial to examine the specific ways in which these cells can target and mitigate the underlying pathological processes.
Preclinical Evidence
Before diving into the mechanisms, we briefly summarize the findings from preclinical studies of MSCs for stroke therapy, particularly using animal models. The majority of these studies utilized murines to develop a model of ischemic stroke via middle cerebral artery occlusion (MCAO). These models are frequently employed under various conditions, including aging, hypertension, and diabetes. Additionally, spontaneously hypertensive rats (SHRs) have been used in studies investigating the association between hypertension and stroke.
Approximately 75% of strokes occur in the elderly population18. Shen et al. (2007) created an ischemic stroke model using 10-12-month-old female retired breeder rats and confirmed the long-term neuroprotective effects of MSCs on ischemic stroke19. It is known that cerebral ischemia is associated with Ca2+-induced calcineurin (CaN) hyperactivation, leading to neuronal apoptosis. Intra-arterial injection of MSCs in a rat model of ischemic stroke was able to reduce CaN expression, rescuing neurons and promoting their survival20.
As hypertension is a common comorbidity in patients with stroke, animal models with hypertensive ischemic stroke have been developed using stroke-prone spontaneously hypertensive rats (SHRSP), which spontaneously develop hypertension and exhibit cerebrovascular pathology akin to human hypertension21. In this model, MSC administration prevented neural cell death through increased expression of anti-apoptotic proteins and improved antioxidative mechanisms22. Furthermore, treatment with maternal-derived MSCs remarkably improved functional outcomes and reduced infarct lesions in hypertensive ischemic mice23.
Diabetes has been established as a significant risk factor for stroke, particularly ischemic stroke. Therefore, it is crucial to study the molecular mechanisms underpinning the increased stroke incidence in diabetes. Hyperglycemic mice exhibited greater infarct volume following permanent MCAO compared to non-hyperglycemic counterparts. This study showed that treatment with human ADSCs did not reduce the lesion size in hyperglycemic stroke rats but significantly improved neurological function24. In addition, intravenous administration of BM-MSCs significantly decreased blood-brain barrier leakage and enhanced vascular and white matter regeneration in type 1 (56) and type 2 (57) diabetic rats, suggesting that MSCs may have neuroprotective effects on stroke patients25.
| Animal species | Comorbidity | Cell source | Cell number | Timing | Key findings | Limitations | References |
| SD | - | BM | 10 5 | 10 days | Neuronal regeneration | Limited to hypoxia-preconditioned MSCs | 26 |
| SD | - | BM | 3x10 6 | 8 days | Reduce infarction volume | Small sample size | 27 |
| Wistar | - | BM | 10 6 | 1,6,24,48 h | Angiogenesis | Short-term follow up | 28 |
| SD | - | BM | 2x10 5 | 1 day | Protect ischemic neurons | Atypical contralateral cell transplantation site | 29 |
| Wistar | Aging | BM | 2x10 6 | 1 day | Long term improvement in functional outcome | Short-term follow up | 19 |
| SD | Aging | BM | 10 5 | 6 h | Elevate the functional outcome | Short-term follow up | 20 |
| SHR | Hypertension | BM | 10 6 | - | Neuroprotective and antioxidant potential | Use of non-autologous MSCs | 22 |
| SHR | Hypertension | Placenta | 10 6 | 8,24 h | Functional recuperation | Lack of long-term cell tracking and survival analysis | 23 |
| SD | Hypertension | Adipose tissue | 10 6 | 48 h | Neurological recuperation | Short-term follow up | 24 |
| Wistar | Diabetes | BM | 5x10 6 | 24 h | Neurorepair recuperation | Short-term follow up | 25 |
Underlying Mechanisms of Mesenchymal Stem Cell for Cognitive Improvement in Stroke
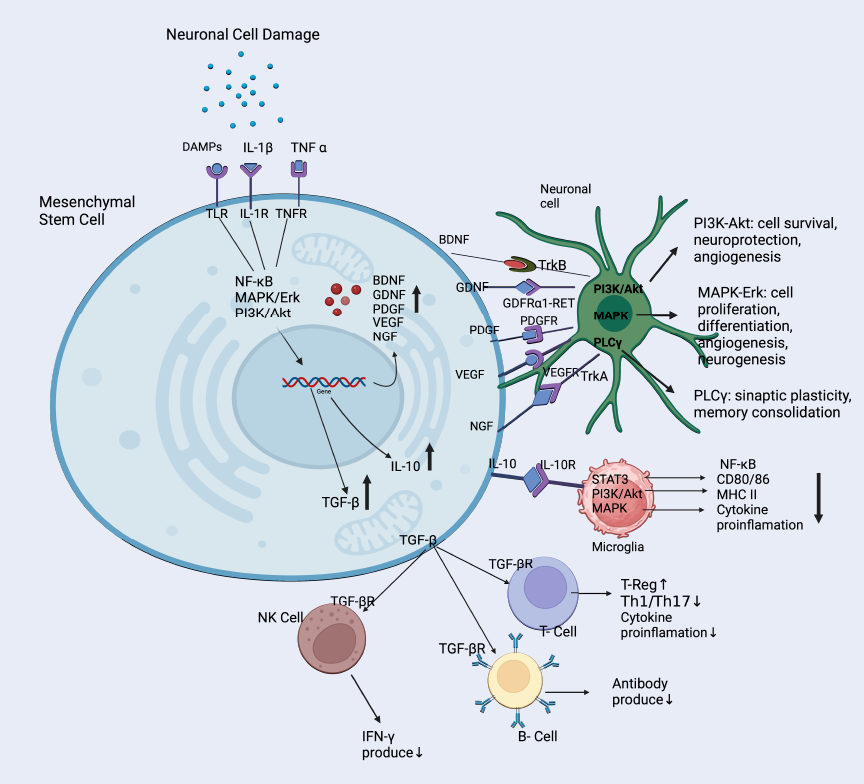
Mesenchymal stem cells are known for their exceptional ability to self-renew and differentiate into various mesenchymal lineages, including adipocytes, chondrocytes, osteoblasts, neurons, and glial cells, under specific conditions30. Mesenchymal stem cells can be obtained from various adult cells, with adipose tissue and bone marrow being the most frequent sources used in MSC-based studies7. The potential of MSCs to enhance cognitive function following stroke is believed to involve multiple mechanisms, including the secretion of trophic factors, immunomodulation, differentiation into neural cell types, promotion of angiogenesis, and replacement of damaged cells, all of which contribute to their therapeutic effects (Figure 1)31. The following sections will explore these mechanisms in more detail.
Secretion of Trophic Factors
Mesenchymal stem cells are a promising option for stroke therapy because they possess the ability to either release or stimulate the secretion of trophic factors, which are crucial in the mechanisms underlying stroke treatment. These trophic factors include neurotrophic factors like brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), nerve growth factor (NGF), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF). These proteins support cell survival, growth, and angiogenesis32. BDNF is a key protein secreted by MSCs that supports neuronal survival and promotes synaptic plasticity. BDNF exerts its effects by binding to the tropomyosin receptor kinase B (TrkB) receptor, triggering signaling pathways like the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways. These pathways regulate gene expression, protein synthesis, and cytoskeletal dynamics, which are vital for synaptic plasticity and cognitive function33. In stroke models, increased BDNF levels following MSC treatment have been associated with improved memory, suggesting its important role in cognitive recovery34.
GDNF is known to support the survival and differentiation of dopaminergic neurons, crucial for cognitive tasks such as attention and executive function35. The mechanism of action involves GDNF binding to the GDNF family receptor α1 (GFRα1), forming a complex with the receptor tyrosine kinase. This complex activates downstream pathways, including PI3K/Akt, MAPK/Erk, and phospholipase C gamma (PLCγ). These pathways promote neuronal survival, growth, synaptic plasticity, and long-term memory consolidation. In stroke animal models, GDNF has been shown to enhance memory and learning36.
In the context of stroke and cognitive function, NGF has been shown to support the survival of cholinergic neurons in the basal forebrain, which are critical for attention, memory, and learning processes. NGF primarily acts by binding to its high-affinity receptor, tropomyosin receptor kinase A (TrkA), which initiates several intracellular signaling cascades. The activation of TrkA leads to the stimulation of three main pathways: the Ras/MAPK pathway, which promotes neuronal survival and differentiation, the PI3K/Akt pathway, which inhibits apoptosis and promotes cell survival, and the PLCγ pathway, which contributes to neuronal plasticity and neurotransmitter release.
VEGF exhibits direct neuroprotective effects by activating anti-apoptotic pathways and promoting angiogenesis. VEGF primarily acts by binding to its receptors, VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1), which are expressed on endothelial cells and neurons. This binding triggers several intracellular signaling cascades, including the PI3K/Akt pathway, which promotes cell survival, and the Ras/MAPK pathway, which stimulates cell proliferation and migration of endothelial cells, leading to the formation of new blood vessels in the ischemic zone37.
PDGF is another important trophic factor secreted by MSCs that has multiple beneficial effects in the context of stroke recovery. PDGF acts by binding to its receptors (PDGFR-α and PDGFR-β), which are receptor tyrosine kinases. This binding triggers receptor dimerization and autophosphorylation, leading to the activation of several signaling pathways, including the PI3K/Akt pathway, the Ras/MAPK pathway, the PLCγ pathway, and the JAK/STAT pathway. These pathways collectively promote cell survival, proliferation, and migration, contributing to neurogenesis and angiogenesis after stroke. PDGF has been shown to enhance cell migration, promote the growth of primary cortical neurons, reduce neuroinflammation, and support angiogenesis and axon growth38.
A study conducted by Nakazaki et al. in 2020 revealed that the infusion of MSCs improved cognitive function in stroke models through the secretion of transforming growth factor-β1 (TGF-β1) and angiopoietin-1 (Ang1). TGF-β1 plays a role in the growth and specialization of endothelial cells and pericytes, which are essential elements of the cerebral microvasculature. Ang1, however, has a function in maintaining the stability of blood vessels and is mostly released by pericytes following the administration of MSCs. Thus, these trophic factors secreted by MSCs play important roles in the improvement of cerebral microvasculature, stabilization of the blood-brain barrier, and reduction of amyloid-β accumulation in the brain. Consequently, this leads to enhanced cognitive function in stroke models39. To better illustrate the various mechanisms involved in MSC therapy for stroke, Figure 1 provides an overview of the therapeutic benefits, key proteins, and mechanisms involved in mesenchymal stem cell therapy for stroke.
Immunomodulation
Mesenchymal stem cells exhibit potent immunomodulatory capabilities, allowing them to effectively regulate immune reactions and facilitate neural restoration and growth40. Two important immunomodulatory molecules secreted by MSCs include interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β). IL-10 production by MSCs is regulated through the STAT3 pathway, while TGF-β production is primarily controlled by the NF-κB and PI3K/Akt pathways. IL-10 binds to the IL-10R complex on immune cells and activates STAT3 signaling to suppress MHC class II expression, co-stimulatory molecules (CD80/86), and pro-inflammatory cytokine production. Similarly, TGF-β signals through the TGF-βR1/R2 complex and activates the SMAD-dependent pathway to induce regulatory T cells and suppress effector immune responses. These paracrines reduce brain inflammation as a result of suppression in the activation and proliferation of pro-inflammatory immune cells, such as T cells and microglia41. In animal models of stroke, MSC transplantation has been observed to elevate levels of IL-10 and TGF-β in the brain, which is associated with enhanced cognitive function42.
Neuroregeneration
While the extent and functional impact of this differentiation in vivo remains under investigation, increasing evidence supports the capacity of MSCs to develop into various neural cell types, including neurons, astrocytes, and oligodendrocytes43. This potentially supports the structural repair of the brain after injury or disease. Research has shown that MSCs can differentiate into neural cell types following induction with specific growth and neurotrophic factors. Previous studies demonstrated that epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) induced the transformation of MSCs into neurospheres, which are subsequently capable of differentiating into neurofilament-positive neurons or glial fibrillary acidic protein (GFAP)-positive glial cells44. Furthermore, brain-derived neurotrophic factor (BDNF) and retinoic acid can stimulate MSCs to become neuron-like cells expressing markers such as β-III tubulin and microtubule-associated protein 2 (MAP2)45. Mesenchymal stem cells can also be induced to differentiate into astrocyte-like cells expressing GFAP using glial cell-derived neurotrophic factor (GDNF). Adipose-derived stem cells (ADSCs) differentiate into neurons or glial cells and express specific molecular markers, such as neuron-specific β-tubulin (Tuj-1), neuron-specific enolase (NSE), MAP2, and neuronal nuclei (NeuN) for neurons, GFAP for astrocytes, and 2',3'-cyclic-nucleotide 3'-phosphodiesterase (CNPase) for oligodendrocytes46.
Enhancement of Angiogenesis
Mesenchymal stem cells have been shown to attenuate cognitive deficits in stroke patients by promoting angiogenesis, which is often disrupted by stroke-induced damage to cerebral vasculature. In a rat stroke model, BMSCs treatment significantly promoted vascular stabilization, which was mediated by increased expression of VEGF/VEGF receptor and angiopoietin-1 (Ang-1)47. Furthermore, conditioned medium (CM) derived from human embryonic stem cell (hESC)-MSC has been shown to accelerate recovery from ischemic infarct in a murine model for cerebral ischemia. The therapeutic effect of CM was attributed to increased blood flow supply through the expression of CD31, a proangiogenic molecule expressed by vascular endothelial cells43.
Challenges and Future Directions
The development of MSC-based therapies for improving cognitive function in stroke patients faces several challenges. One of the primary obstacles is the limited data on the mechanisms by which MSCs exert their therapeutic effects on the brain. Further studies to gain a deeper understanding of the mechanistic pathways underlying the neuroprotective and neuroregenerative effects of MSCs are warranted48. Additionally, the optimal dosage, timing, and route of administration for MSC therapy in stroke patients remain to be determined49. The standardization of cell isolation, culture, and characterization protocols is also crucial to ensure the reproducibility and reliability of the results50. To address these challenges, future research should focus on elucidating specific molecular mechanisms, particularly the roles of key proteins such as BDNF, GDNF, and their respective receptors (TrkB and GFRα1) in MSC-mediated cognitive improvement. Investigating downstream signaling pathways like PI3K/Akt and MAPK could reveal potential targets for enhancing MSC efficacy.
Moreover, innovative approaches such as combining MSC therapy with rehabilitation techniques or utilizing advanced delivery methods (e.g., engineered scaffolds or nanoparticles) could optimize treatment protocols. Exploring the impact of different culture conditions, such as hypoxic preconditioning, on MSC therapeutic potential may also lead to improved outcomes.
Despite these challenges, the potential of MSC-based therapies to enhance cognitive function in stroke patients presents exciting opportunities for future research and development. The findings of this study have important implications for clinical practice, as they suggest that MSC therapy could be a promising adjuvant treatment for cognitive impairment following stroke. Future directions in this field may include the development of personalized cell-based therapies tailored to individual patient needs, as well as the exploration of combination therapies that integrate MSCs with other neuroprotective or neuroregenerative agents. Moreover, cohort prospective studies are necessary to evaluate the sustainability of cognitive benefits and monitor any potential adverse effects associated with MSC therapy in patients with stroke.
Abbreviations
ADSCs - Adipose-Derived Stem Cells, Ang1 - Angiopoietin-1, BDNF - Brain-Derived Neurotrophic Factor, BM-MSCs - Bone Marrow Mesenchymal Stem Cells, BMSCs - Bone Marrow Stem Cells, CaN - Calcineurin, CM - Conditioned Medium, CNPase - 2',3'-Cyclic-nucleotide 3'-Phosphodiesterase, EGF - Epidermal Growth Factor, GFAP - Glial Fibrillary Acidic Protein, GDNF - Glial Cell Line-Derived Neurotrophic Factor, GFRα1 - GDNF Family Receptor α1, hESC - Human Embryonic Stem Cell, IL-10 - Interleukin-10, MAP2 - Microtubule-Associated Protein 2, MAPK - Mitogen-Activated Protein Kinase, MCAO - Middle Cerebral Artery Occlusion, MSCs - Mesenchymal Stem Cells, NeuN - Neuronal Nuclei, NF-κB - Nuclear Factor kappa-light-chain-enhancer of activated B cells, NGF - Nerve Growth Factor, NSE - Neuron-Specific Enolase, PDGF - Platelet-Derived Growth Factor, PI3K - Phosphatidylinositol 3-Kinase, PLCγ - Phospholipase C Gamma, PSCI - Post-Stroke Cognitive Impairment, SHRs - Spontaneously Hypertensive Rats, SHRSP - Stroke-prone Spontaneously Hypertensive Rats, SMAD - A family of proteins involved in signal transduction, TGF-β - Transforming Growth Factor-beta, TGF-β1 - Transforming Growth Factor-β1, TrkB - Tropomyosin Receptor Kinase B, Tuj-1 - Neuron-specific β-Tubulin, VEGF - Vascular Endothelial Growth Factor
Acknowledgments
None.
Author’s contributions
All authors contributed equally to this work, and they have read and approved the final manuscript.
Funding
This study was funded by Research Grants from The Directorate of Research, Technology, and Community Service (DRTPM), Indonesian Ministry of Education, Culture, Research, and Technology 2023 (Contract no: 15456/UN19.5.1.3/AL.04/2023)
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Kalladka
D.,
Muir
K.W.,
Brain repair: cell therapy in stroke. Stem Cells and Cloning : Advances and Applications.
2014;
7
:
31-44
.
View Article PubMed Google Scholar -
Roy-O'Reilly
M.,
McCullough
L.D.,
Sex differences in stroke: the contribution of coagulation. Experimental Neurology.
2014;
259
:
16-27
.
View Article PubMed Google Scholar -
Li
W.,
Shi
L.,
Hu
B.,
Hong
Y.,
Zhang
H.,
Li
X.,
Mesenchymal Stem Cell-Based Therapy for Stroke: Current Understanding and Challenges. Frontiers in Cellular Neuroscience.
2021;
15
:
628940
.
View Article PubMed Google Scholar -
Bhaskar
S.,
Stanwell
P.,
Cordato
D.,
Attia
J.,
Levi
C.,
Reperfusion therapy in acute ischemic stroke: dawn of a new era?. BMC Neurology.
2018;
18
(1)
:
8
.
View Article PubMed Google Scholar -
Lyden
J.,
Grant
S.,
Ma
T.,
Altered metabolism for neuroprotection provided by mesenchymal stem cells. Brain Circulation.
2019;
5
(3)
:
140-4
.
View Article PubMed Google Scholar -
Elendu
C.,
Amaechi
D.C.,
Elendu
T.C.,
Ibhiedu
J.O.,
Egbunu
E.O.,
Ndam
A.R.,
Stroke and cognitive impairment: understanding the connection and managing symptoms. Annals of Medicine and Surgery (London).
2023;
85
(12)
:
6057-66
.
View Article PubMed Google Scholar -
Sui
B.D.,
Zheng
C.X.,
Li
M.,
Jin
Y.,
Hu
C.H.,
Epigenetic Regulation of Mesenchymal Stem Cell Homeostasis. Trends in Cell Biology.
2020;
30
(2)
:
97-116
.
View Article PubMed Google Scholar -
Chrostek
M.R.,
Fellows
E.G.,
Crane
A.T.,
Grande
A.W.,
Low
W.C.,
Efficacy of stem cell-based therapies for stroke. Brain Research.
2019;
1722
:
146362
.
View Article PubMed Google Scholar -
Barbay
M.,
Diouf
M.,
Roussel
M.,
Godefroy
O.,
study group
GRECOGVASC,
Systematic review and meta-analysis of prevalence in post-stroke neurocognitive disorders in hospital-based studies. Vol. 46. Dementia and Geriatric Cognitive Disorders.
2018;
46
(5-6)
:
322-34
.
View Article PubMed Google Scholar -
Dichgans
M.,
Leys
D.,
Vascular Cognitive Impairment. Circulation Research.
2017;
120
(3)
:
573-91
.
View Article PubMed Google Scholar -
Otero
T.M.,
Barker
L.A.,
The frontal lobes and executive functioning. InHandbook of executive functioning 2013 Sep 12 (pp. 29-44). New York, NY: Springer New York. 2014
.
View Article Google Scholar -
Zhao
L.,
Biesbroek
J.M.,
Shi
L.,
Liu
W.,
Kuijf
H.J.,
Chu
W.W.,
Strategic infarct location for post-stroke cognitive impairment: A multivariate lesion-symptom mapping study. Journal of Cerebral Blood Flow and Metabolism.
2018;
38
(8)
:
1299-311
.
View Article PubMed Google Scholar -
Mijajlović
M.D.,
Pavlović
A.,
Brainin
M.,
Heiss
W.D.,
Quinn
T.J.,
Ihle-Hansen
H.B.,
Post-stroke dementia - a comprehensive review. BMC Medicine.
2017;
15
(1)
:
11
.
View Article PubMed Google Scholar -
Ritter
A.,
Pillai
J.A.,
Treatment of Vascular Cognitive Impairment. Current Treatment Options in Neurology.
2015;
17
(8)
:
367
.
View Article PubMed Google Scholar -
Zucchella
C.,
Capone
A.,
Codella
V.,
De Nunzio
A.M.,
Vecchione
C.,
Sandrini
G.,
Cognitive rehabilitation for early post-surgery inpatients affected by primary brain tumor: a randomized, controlled trial. Journal of Neuro-Oncology.
2013;
114
(1)
:
93-100
.
View Article PubMed Google Scholar -
Lu
Q.,
Yu
A.,
Pu
J.,
Chen
D.,
Zhong
Y.,
Bai
D.,
Post-stroke cognitive impairment: exploring molecular mechanisms and omics biomarkers for early identification and intervention. Frontiers in Molecular Neuroscience.
2024;
17
:
1375973
.
View Article PubMed Google Scholar -
Zhao
Y.,
Peng
Y.,
Pan
Y.,
Lv
Y.,
Zhou
H.,
Wu
J.,
The role of ventral hippocampal-medial prefrontal glutamatergic pathway on the non-affected side in post-stroke cognitive impairment. Brain Research.
2024;
1845
:
149168
.
View Article PubMed Google Scholar -
Simmons
C.A.,
Poupore
N.,
Nathaniel
T.I.,
Age Stratification and Stroke Severity in the Telestroke Network. Journal of Clinical Medicine.
2023;
12
(4)
:
1519
.
View Article PubMed Google Scholar -
Shen
L.H.,
Li
Y.,
Chen
J.,
Zacharek
A.,
Gao
Q.,
Kapke
A.,
Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. Journal of Cerebral Blood Flow and Metabolism.
2007;
27
(1)
:
6-13
.
View Article PubMed Google Scholar -
Saraf
J.,
Sarmah
D.,
Vats
K.,
Kaur
H.,
Pravalika
K.,
Wanve
M.,
Intra-arterial stem cell therapy modulates neuronal calcineurin and confers neuroprotection after ischemic stroke. The International Journal of Neuroscience.
2019;
129
(10)
:
1039-44
.
View Article PubMed Google Scholar -
Cipolla
M.J.,
Liebeskind
D.S.,
Chan
S.L.,
The importance of comorbidities in ischemic stroke: impact of hypertension on the cerebral circulation. Journal of Cerebral Blood Flow and Metabolism.
2018;
38
(12)
:
2129-49
.
View Article PubMed Google Scholar -
Calió
M.L.,
Marinho
D.S.,
Ko
G.M.,
Ribeiro
R.R.,
Carbonel
A.F.,
Oyama
L.M.,
Transplantation of bone marrow mesenchymal stem cells decreases oxidative stress, apoptosis, and hippocampal damage in brain of a spontaneous stroke model. Free Radical Biology & Medicine.
2014;
70
:
141-54
.
View Article PubMed Google Scholar -
Kranz
A.,
Wagner
D.C.,
Kamprad
M.,
Scholz
M.,
Schmidt
U.R.,
Nitzsche
F.,
Transplantation of placenta-derived mesenchymal stromal cells upon experimental stroke in rats. Brain Research.
2010;
1315
:
128-36
.
View Article PubMed Google Scholar -
Gómez-de Frutos
M.C.,
Laso-García
F.,
Diekhorst
L.,
Otero-Ortega
L.,
Fuentes
B.,
Jolkkonen
J.,
consortium
RESSTORE,
Intravenous delivery of adipose tissue-derived mesenchymal stem cells improves brain repair in hyperglycemic stroke rats. Stem Cell Research {&}amp; Therapy.
2019;
10
(1)
:
212
.
View Article PubMed Google Scholar -
Cui
C.,
Ye
X.,
Chopp
M.,
Venkat
P.,
Zacharek
A.,
Yan
T.,
miR-145 Regulates Diabetes-Bone Marrow Stromal Cell-Induced Neurorestorative Effects in Diabetes Stroke Rats. Stem Cells Translational Medicine.
2016;
5
(12)
:
1656-67
.
View Article PubMed Google Scholar -
Son
J.W.,
Park
J.,
Kim
Y.E.,
Ha
J.,
Park
D.W.,
Chang
M.S.,
Glia-like cells from late-passage human MSCs protect against ischemic stroke through IGFBP-4. Molecular Neurobiology.
2019;
56
(11)
:
7617-30
.
View Article PubMed Google Scholar -
Toyoshima
A.,
Yasuhara
T.,
Kameda
M.,
Morimoto
J.,
Takeuchi
H.,
Wang
F.,
Intra-arterial transplantation of allogeneic mesenchymal stem cells mounts neuroprotective effects in a transient ischemic stroke model in rats: analyses of therapeutic time window and its mechanisms. PLoS One.
2015;
10
(6)
:
e0127302
.
View Article PubMed Google Scholar -
Moisan
A.,
Favre
I.,
Rome
C.,
De Fraipont
F.,
Grillon
E.,
Coquery
N.,
Intravenous injection of clinical grade human MSCs after experimental stroke: functional benefit and microvascular effect. Cell Transplantation.
2016;
25
(12)
:
2157-71
.
View Article PubMed Google Scholar -
Hu
Y.,
Chen
W.,
Wu
L.,
Jiang
L.,
Qin
H.,
Tang
N.,
Hypoxic preconditioning improves the survival and neural effects of transplanted mesenchymal stem cells via CXCL12/CXCR4 signalling in a rat model of cerebral infarction. Cell Biochemistry and Function.
2019;
37
(7)
:
504-15
.
View Article PubMed Google Scholar -
Li
S.N.,
Wu
J.F.,
TGF-β/SMAD signaling regulation of mesenchymal stem cells in adipocyte commitment. Stem Cell Research & Therapy.
2020;
11
(1)
:
41
.
View Article PubMed Google Scholar -
Dabrowska
S.,
Andrzejewska
A.,
Lukomska
B.,
Janowski
M.,
Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. Journal of Neuroinflammation.
2019;
16
(1)
:
178
.
View Article PubMed Google Scholar -
Zhang
Y.,
Dong
N.,
Hong
H.,
Qi
J.,
Zhang
S.,
Wang
J.,
Mesenchymal stem cells: therapeutic mechanisms for stroke. International Journal of Molecular Sciences.
2022;
23
(5)
:
2550
.
View Article PubMed Google Scholar -
Kowiański
P.,
Lietzau
G.,
Czuba
E.,
Waśkow
M.,
Steliga
A.,
Moryś
J.,
BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cellular and Molecular Neurobiology.
2018;
38
(3)
:
579-93
.
View Article PubMed Google Scholar -
Jeong
C.H.,
Kim
S.M.,
Lim
J.Y.,
Ryu
C.H.,
Jun
J.A.,
Jeun
S.S.,
Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. BioMed Research International.
2014;
2014
:
129145
.
View Article PubMed Google Scholar -
Kramer
E.R.,
Liss
B.,
GDNF-Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Letters.
2015;
589
(24PartA)
:
3760-72
.
View Article PubMed Google Scholar -
Palasz
E.,
Wilkaniec
A.,
Stanaszek
L.,
Andrzejewska
A.,
Adamczyk
A.,
Glia-Neurotrophic Factor Relationships: Possible Role in Pathobiology of Neuroinflammation-Related Brain Disorders. International Journal of Molecular Sciences.
2023;
24
(7)
:
6321
.
View Article PubMed Google Scholar -
Wittko-Schneider
I.M.,
Schneider
F.T.,
Plate
K.H.,
Brain homeostasis: VEGF receptor 1 and 2-two unequal brothers in mind. Cellular and Molecular Life Sciences.
2013;
70
(10)
:
1705-25
.
View Article PubMed Google Scholar -
Li
D.,
Huang
L.T.,
Zhang
C.P.,
Li
Q.,
Wang
J.H.,
Insights Into the Role of Platelet-Derived Growth Factors: Implications for Parkinson's Disease Pathogenesis and Treatment. Frontiers in Aging Neuroscience.
2022;
14
:
890509
.
View Article PubMed Google Scholar -
Nakazaki
M.,
Sasaki
M.,
Kataoka-Sasaki
Y.,
Oka
S.,
Suzuki
J.,
Sasaki
Y.,
Intravenous infusion of mesenchymal stem cells improves impaired cognitive function in a cerebral small vessel disease model. Neuroscience.
2019;
408
:
361-77
.
View Article PubMed Google Scholar -
Li
N.,
Hua
J.,
Interactions between mesenchymal stem cells and the immune system. Cellular and Molecular Life Sciences.
2017;
74
(13)
:
2345-60
.
View Article PubMed Google Scholar -
Jiang
W.,
Xu
J.,
Immune modulation by mesenchymal stem cells. Cell proliferation.
2020;
53
(1)
:
e12712
.
View Article Google Scholar -
Li
Y.,
Huang
J.,
Wang
J.,
Xia
S.,
Ran
H.,
Gao
L.,
Human umbilical cord-derived mesenchymal stem cell transplantation supplemented with curcumin improves the outcomes of ischemic stroke via AKT/GSK-3β/β-TrCP/Nrf2 axis. Journal of Neuroinflammation.
2023;
20
(1)
:
49
.
View Article PubMed Google Scholar -
Bao
C.S.,
Li
X.L.,
Liu
L.,
Wang
B.,
Yang
F.B.,
Chen
L.G.,
Transplantation of Human umbilical cord mesenchymal stem cells promotes functional recovery after spinal cord injury by blocking the expression of IL-7. European Review for Medical and Pharmacological Sciences.
2018;
22
(19)
:
6436-47
.
View Article PubMed Google Scholar -
Marei
H.E.,
El-Gamal
A.,
Althani
A.,
Afifi
N.,
Abd-Elmaksoud
A.,
Farag
A.,
Cholinergic and dopaminergic neuronal differentiation of human adipose tissue derived mesenchymal stem cells. Journal of Cellular Physiology.
2018;
233
(2)
:
936-45
.
View Article PubMed Google Scholar -
Darkazalli
A.,
Ismail
A.A.,
Abad
N.,
Grant
S.C.,
Levenson
C.W.,
Use of human mesenchymal stem cell treatment to prevent anhedonia in a rat model of traumatic brain injury. Restorative Neurology and Neuroscience.
2016;
34
(3)
:
433-41
.
View Article PubMed Google Scholar -
Blecker
D.,
Elashry
M.I.,
Heimann
M.,
Wenisch
S.,
Arnhold
S.,
New insights into the neural differentiation potential of canine adipose tissue-derived mesenchymal stem cells. Anatomia, Histologia, Embryologia.
2017;
46
(3)
:
304-15
.
View Article PubMed Google Scholar -
Zacharek
A.,
Chen
J.,
Cui
X.,
Li
A.,
Li
Y.,
Roberts
C.,
Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. Journal of Cerebral Blood Flow and Metabolism.
2007;
27
(10)
:
1684-91
.
View Article PubMed Google Scholar -
Krause
M.,
Phan
T.G.,
Ma
H.,
Sobey
C.G.,
Lim
R.,
Cell-based therapies for stroke: are we there yet?. Frontiers in Neurology.
2019;
10
:
656
.
View Article PubMed Google Scholar -
Sarmah
D.,
Agrawal
V.,
Rane
P.,
Bhute
S.,
Watanabe
M.,
Kalia
K.,
Mesenchymal Stem Cell Therapy in Ischemic Stroke: A Meta-analysis of Preclinical Studies. Clinical Pharmacology and Therapeutics.
2018;
103
(6)
:
990-8
.
View Article PubMed Google Scholar -
Kim
S.Y.,
Phan
T.H.,
Limantoro
C.,
Kalionis
B.,
Chrzanowski
W.,
Isolation and characterization of extracellular vesicles from mesenchymal stromal cells. Methods in Molecular Biology (Clifton, N.J.).
2019;
2029
:
15-23
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 12 (2024)
Page No.: 7015-7023
Published on: 2024-12-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 1219 times
- PDF downloaded - 389 times
- XML downloaded - 68 times
 Biomedpress
Biomedpress



