Optimizing in vitro posterior capsule opacification models in lens epithelial cells: A systematic review
- Jaksa Agung Suprapto No. 2 Malang, East Java, Indonesia
- Jl. Veteran, Ketawanggede, Malang City, East Java, Indonesia
Abstract
Posterior capsule opacification (PCO) remains the most common complication following cataract surgery, and transforming growth factor-beta 2 (TGF-β2) is a key mediator of its pathogenesis. This systematic review evaluates the culture media, TGF-β2 concentrations, and incubation periods used in in vitro PCO models to identify conditions that favour reproducibility. A comprehensive literature search was performed in PubMed, ScienceDirect, SpringerLink, Taylor & Francis and SAGE Journals using the MeSH terms “posterior capsule opacification” OR “posterior capsular opacification” OR “capsule opacifications” OR “secondary cataract” OR “PCO” AND “lens epithelial cells” AND “TGF-β2” OR “epithelial–mesenchymal transition (EMT)”. Original full-text English articles published between 1 June 2014 and 30 July 2024 were included; abstracts, preprints, and non-English reports were excluded. Sixteen eligible studies were retrieved. Three studies employed 5 ng/mL TGF-β2, whereas seven used 10 ng/mL; both doses induced morphological changes in lens epithelial cells. Nine studies utilised Dulbecco’s modified Eagle medium (DMEM). Most experiments (n = 8) incubated cells for 24 h, whereas others extended incubation to ≥ 48 h depending on study design. Collectively, 10 ng/mL TGF-β2, DMEM and an incubation period of 24–48 h represent the most frequently used conditions and may improve the reproducibility of in vitro PCO models, ultimately guiding the development of interventions to prevent PCO after cataract surgery.
INTRODUCTION
Cataracts are the leading cause of blindness worldwide, and surgical extraction remains the standard treatment. Despite technological advances in cataract surgery, posterior capsule opacification (PCO) continues to be a common postoperative complication 1. PCO not only reduces visual acuity but also degrades visual quality, producing symptoms such as halos and diminished contrast sensitivity. It affects approximately 20–40 % of adult and almost 100 % of paediatric cataract patients after surgery, leading to secondary vision loss 2. PCO develops mainly from the proliferation and migration of residual lens epithelial cells (LECs) stimulated by postoperative inflammation and wound-healing responses. These LECs subsequently undergo transdifferentiation or epithelial–mesenchymal transition (EMT) into fibroblast-like and lens-fiber-like cells, processes driven by growth factors and extracellular-matrix (ECM)-related proteins 3. Although PCO is clinically important, there is currently no standardized protocol or standard operating procedure (SOP) for generating in-vitro PCO models. This lack of uniformity hampers the development of preventive measures and research strategies. Establishing standardized model systems would streamline PCO research by minimizing variability in culture media and methodology and by reducing experimental time 1.
Another process initiated during the inflammatory response and wound-healing cascade is the migration of lens epithelial cells (LECs) to the posterior capsule. LECs located at the lens equator can transdifferentiate into cells resembling normal lens fibres, whereas those that remain on the anterior capsule may differentiate into myofibroblasts, thereby promoting posterior capsule opacification (PCO) 4. A hallmark of PCO formation is the induction of transforming growth factor-beta (TGF-β). During PCO development, TGF-β signals via the SMAD (small mothers against decapentaplegic) cascade in addition to several SMAD-independent pathways. Among the three isoforms, TGF-β2 is the most biologically potent and is the predominant isoform in the eye. TGF-β is present in a latent form within the aqueous humour but is activated after cataract surgery. Activation of TGF-β initiates LEC proliferation, migration and epithelial-to-mesenchymal transition (EMT) 5. Prior reviews have examined PCO broadly, encompassing its pathophysiology, surgical techniques, intraocular lens (IOL) designs and pharmacological prophylaxis 1,4. Although models were occasionally discussed, critical technical variables—including culture medium composition, TGF-β2 concentration and incubation duration—were not systematically analysed. Standardising an methodology for inducing PCO is essential for progress toward preventive strategies. Effective models enable a deeper understanding of the cellular mechanisms underlying PCO, allowing investigators to evaluate candidate interventions under controlled conditions. This systematic review therefore synthesises recent evidence to propose optimised parameters for reproducible PCO models.
MATERIALS AND METHODS
Research Question
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement. The research questions were formulated using the Population, Intervention, Comparison, and Outcome (PICO) framework, in which the population corresponded to the investigated cell type; the intervention entailed TGF-β2 administration; the comparison involved an appropriate control group; and the outcomes of interest were the onset of posterior capsular opacification (PCO), the concentration of TGF-β2 required to induce PCO, and the composition of the cell-culture medium.
Literature search and eligibility criteria
We included population-based studies that reported the use of TGF-β2 in posterior capsule opacification (PCO) modeling using lens epithelial cells. Eligibility criteria were predefined and comprised original , full-text articles published in English between 1 June 2014 and 30 July 2024. Abstracts, preprints, and non-English publications were excluded. Studies employing wounded capsular-bag models or administering additional treatments prior to TGF-β2 exposure were also excluded, as such models represent a more complex tissue system with intrinsic wound-healing responses that differ from those observed in simplified cell-culture assays. The review protocol was prospectively registered on the Open Science Framework (OSF) (DOI: ), because PROSPERO does not accept registrations of systematic reviews limited to studies.
Search strategy and screening
Two investigators (LRW and IK) independently extracted study data from the PubMed, ScienceDirect, SpringerLink, Taylor & Francis, and SAGE Journals databases. A comprehensive search strategy based on Medical Subject Headings (MeSH) terms was implemented in each database as follows: (“posterior capsule opacification” OR “posterior capsular opacification” OR “capsule opacifications” OR “secondary cataract” OR “PCO”) AND (“lens epithelial cells”) AND (“TGF-β2” OR “epithelial–mesenchymal transition (EMT)”). The search covered the period from 1 June 2014 to 15 July 2024. Three additional researchers (H.S., S.W., and H.K.P.) resolved any discrepancies. The literature search was finalised at the end of September 2024.
Data extraction
Studies were classified according to their respective research methodologies. Two investigators independently extracted data, recording the following variables: title, authors, year of publication, cell type, culture medium, TGF-β2 dose, PCO timing, procedures employed, and both quantitative and qualitative outcomes.
Data synthesis
The data were synthesized qualitatively. Findings from 16 in vitro studies examining TGF-β2–induced posterior capsular opacification (PCO) changes were summarized to highlight molecular and cellular responses associated with the treatment, specifically whether TGF-β2 exposure up-regulated epithelial-to-mesenchymal transition (EMT) markers—α-SMA, fibronectin, collagen I, vimentin, and N-cadherin—and/or down-regulated epithelial markers such as E-cadherin and ZO-1. When available, fold-change values were extracted and mapped to a semi-quantitative EMT score according to predefined criteria: mild (+) = 1–2 markers increased <2-fold with minimal morphological change; moderate (++) = 2–3 markers increased 2–4-fold or clear qualitative EMT features; marked (+++) = ≥3 markers increased ≥4-fold or consistent EMT evidence across multiple assays. Two reviewers (I.K. and L.R.W.) independently applied the scoring criteria, and disagreements were resolved through consensus with a third reviewer.
Assessment of Methodological Quality
The methodological quality of the included in-vitro studies was evaluated with the QUIN tool. Although the QUIN tool was originally created for dental studies, it was chosen here because, to our knowledge, no validated risk-of-bias instrument exists for ophthalmic cell-culture research. Several of its assessment domains—sample selection, randomisation, blinding, outcome reporting, and statistical analysis—are broadly applicable to laboratory-based experimental studies. For each study, twelve criteria were rated “Yes” (2 points), “Partial” (1 point), or “No” (0 points), yielding a maximum score of 24. For the sampling method, a score of 0 was assigned when the procedure was not reported, 1 when partially described, and 2 when fully described (including cell source, passage number, and handling). For randomisation, all studies received a score of 0, since none explicitly mentioned random allocation of dishes or wells, despite this being technically possible in cell culture. Overall percentage scores were calculated as (Total Score ÷ 24) × 100. Based on these percentages, studies were classified as having low (>70%), medium (50–70%), or high (<50%) risk of bias 6. Detailed domain-level scores for all included studies are presented in Appendix 1 (Supplementary Material). The evaluations were carried out independently by two authors (I.K. and L.R.W.), and discrepancies were resolved through discussion, with a third reviewer (H.S.) acting as arbiter when required.
RESULT
Included Studies
The study selection process was conducted in accordance with the PRISMA 2020 flowchart (Figure 1). Database searching yielded 287 records: PubMed (n = 202), ScienceDirect (n = 85), SpringerLink (n = 0), Taylor & Francis (n = 2), and SAGE Journals (n = 3). After removal of 139 duplicates and 3 conference abstracts, 145 records remained. Title and abstract screening eliminated 128 records. During full-text assessment, 3 articles were excluded because their samples originated from injured capsular tissue, and 3 were excluded because the cells had received additional treatments before TGF-β₂ exposure. Detailed reasons for exclusion are provided in Supplementary Table S1. Ultimately, 16 studies met the inclusion criteria and were incorporated into the review (
Baseline Characteristic of Included Studies
The selected studies were further analyzed by extracting key information related to PCO modelling, as presented in
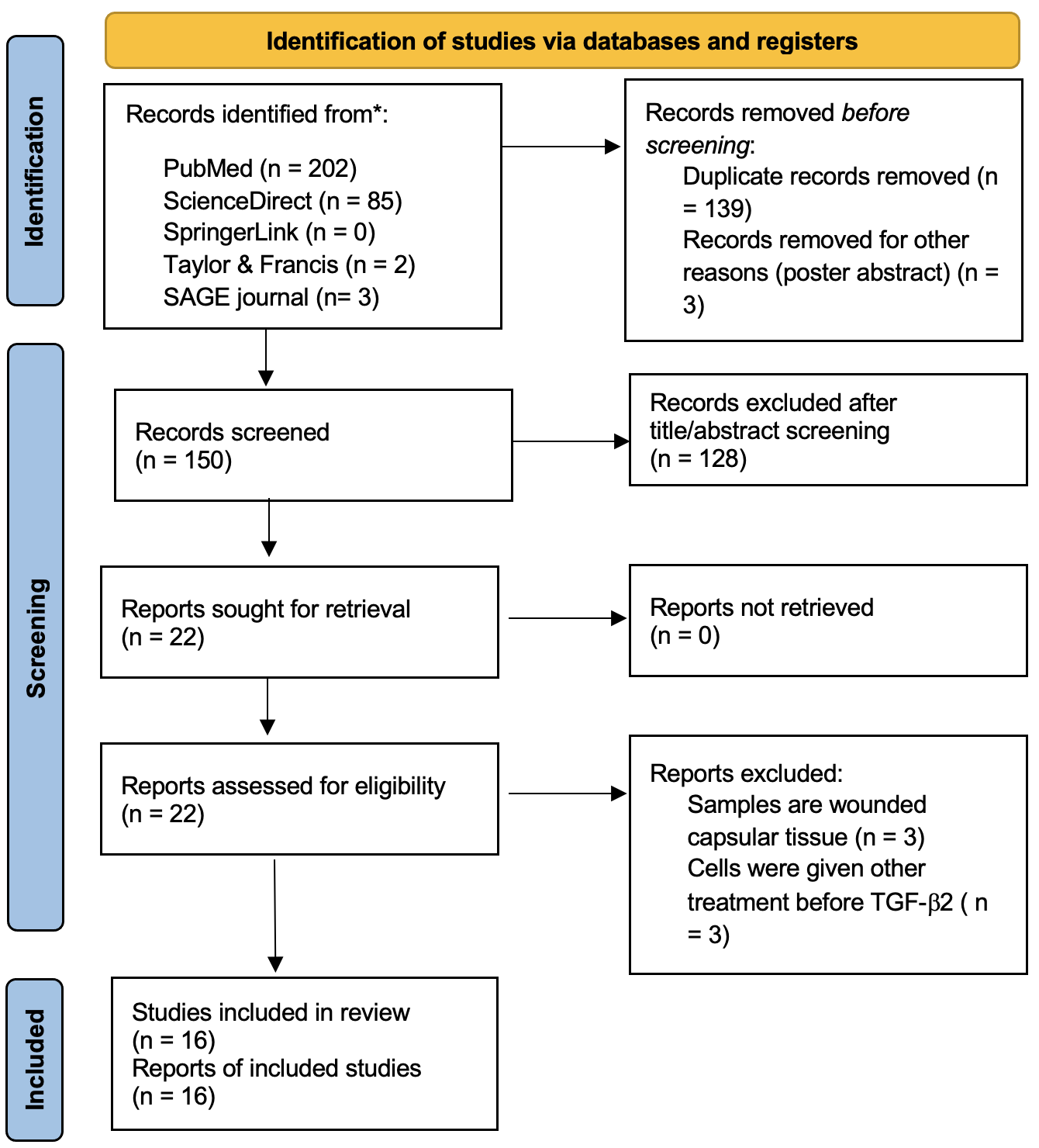
PRISMA flowchart detailing the search and selection process.
Risk of Bias Assessment
The methodological quality of the 16 included studies was assessed using the QUIN tool, which evaluates 12 domains, including sample selection, randomisation, blinding, outcome measurement, and statistical analysis; each domain is assigned 0–2 points. All investigations were therefore classified as having a low risk of bias. This uniform rating likely reflects the standardised reporting typically observed in in-vitro lens epithelial cell experiments, where core procedures are largely harmonised. Conversely, the clustering of scores also highlights a limitation of the QUIN tool, namely its reduced ability to discriminate between methodological strengths and weaknesses when studies follow similar laboratory workflows. The individual QUIN scoring sheets for each of the 12 domains per study are available in Appendix 1 (Supplementary Material) for transparency.
Risk Bias of Assessment using QUIN Tool (n=16)
| First Author | Aim | Sample Size | Sampling Method | Control Group | Methodology | Operator Info | Randomization | Outcome Measures | Assessor Info | Blinding | Stats Analysis | Result Report | Bias Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xie, 2022 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | Low |
| Zhang, 2018 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | Low |
| Cui, 2022 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | Low |
| Huang, 2020 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | Low |
| Han, 2019 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 0 | 0 | 2 | 2 | Low |
| Yu, 2021 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | Low |
| Raghavan and Nagaraj, 2016 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | Low |
| Raghavan, 2016 | 2 | 0 | 2 | 2 | 1 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | Low |
| Fan, 2024 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 2 | 2 | Low |
| Xiong, 2022 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | Low |
| Wang and Zheng, 2022 | 2 | 1 | 2 | 2 | 2 | 1 | 0 | 2 | 1 | 0 | 2 | 2 | Low |
| Smith, 2019 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 2 | 2 | Low |
| Nahomi, 2016 | 2 | 0 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | Low |
| Sun, 2021 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | Low |
| Zhang, 2017 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 2 | 2 | Low |
| Wang, 2021 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 2 | 2 | Low |
Characteristics of included studies with (medium, TGF-β2 dose, incubation time) and EMT responses (n=16 experimental
| Author | Cell type | Culture Medium | TGF-β2 doses (ng/mL) | PCO timing (h) | Upregulation of EMT Markers Expression | Downregulation of Epithelial Markers | EMT outcome strength (+/++/+++) |
|---|---|---|---|---|---|---|---|
| Xie | HLE cell lines (SRA01-04) | DMEM | 5 | 48-72 | α-SMA, FN, Col I, and Agn. | E-cad, ZO-1 | +++ |
| Zhang | HLE cell lines (SRA01-04) | DMEM | 10 | 24-48 | VIM and α-SMA | E-cad, ZO-1 | ++ |
| Cui | HLE cell lines (SRA01-04) | DMEM | 0, 1, 5, 10, 15, 20 -> autophagy started in 10 | 24 | α-SMA, VIM, autophagy | E-cad | +++ |
| Huang | HLE cell line (SRA01/04) | DMEM | 10 | 24 | mRNA, α-SMA, FN | E-cad, Cx43 | +++ |
| Han | LECs | Epithelial cell medium | 10 | 24 | Snail mRNA, FN, VIM, Col I | E-cad | +++ |
| Yu and Li, 2021 | HLE cell line SRA1/04 cells | DMEM with 10% FBS | 0, 1, 5, 10 | 24-48 | α-SMA, VIM | E-cad | ++ |
| Xiong | HLE cell line (SRA01/04) | DMEM with 10% FBS | 10 | 48 | FN, α-SMA, N-cad | E-cad | +++ |
| Wang and Zheng, 2022 | HLE cells (SRA01/04) | DMEM | 0, 3, 6, 9 | 24 | α-SMA, FN, Col I, N-cad, MMP-2 | E-cad | +++ |
| Smith, Eldred and Wormstone, 2019 | HLE cell line (FHL124) | EMEM with 5% fetal calf serum | 10 | 24-48 | ACTA2, α-SMA, FN | Not reported | ++ |
| Nahomi, et. al., 2016 | Multiple cell tyes (FHL124, bovine explants, mouse LECs, HeLa) | Serum-free medium | 20 | 24-48 | mRNA expression of α-SMA, FN, and β1 integrin | E-cad | +++ |
| Zhang | HLE cell line (HLE B-3 cell line) | DMEM with 15% fetal bovine serum | 10 | 24 | α-SMA, VIM | E-cad | +++ |
| Wang | HLE cell line | 10% FBS-DMEM/F-12 medium | 0, 2, 5, 10, 20 | 24 | Col I, α-SMA, FN | E-cad | +++ |
| Sun | LECs | MEM with 10% fetal bovine serum | 2.5, 5, 10 | Migration: 36 h; EMT marker changes: 48–96 h (2–4 days) | α-SMA, FN, and LC3-II | E-cad | +++ |
| Raghavan and Nagaraj, 2016 | HLE cell line (FHL124 cells) | AGE-modified or unmodified BME | 10 | 24 | NF-kB, ROS levels | Not reported | ++ |
| Raghavan | Human lens capsules | AGE-modified or unmodified BME in 20% FBS-MEM | 10 | 24 | αSMA, CTGF, and integrin αV | Not reported | +++ |
| Fan | pLECs SRA01/04 cell line | Serum-free medium | 5 | 48 | EMT-related DEGs | Not reported | +++ |
To visualize heterogeneity in EMT induction, we constructed a heat-map-style table (Figure 2) summarizing cell type, TGF-β2 dose, incubation time, and EMT outcome strength across the included studies. Each row represents a study, whereas the columns employ categorical color-coding to facilitate rapid visual comparison between models. TGF-β2 doses were classified into three categories: low (0–5 ng/mL) shown in yellow, medium (5–10 ng/mL) shown in orange, and high (15–20 ng/mL) shown in dark orange. Incubation time was similarly categorized as short (≤ 24 h) in light turquoise, intermediate (24–48 h) in mid-blue, and long (> 48 h) in dark blue. EMT strength was depicted using a purple gradient, with weak (+) coded as dark violet, moderate (++) as lavender, and strong (+++) as pink.
This visual synopsis highlights the consistent induction of strong EMT responses (+++) across most studies, particularly at medium to high TGF-β2 doses and incubation periods exceeding 24 h. A minority of investigations employing lower doses or alternative culture systems (e.g., FHL124 cells) exhibited moderate rather than strong EMT outcomes.
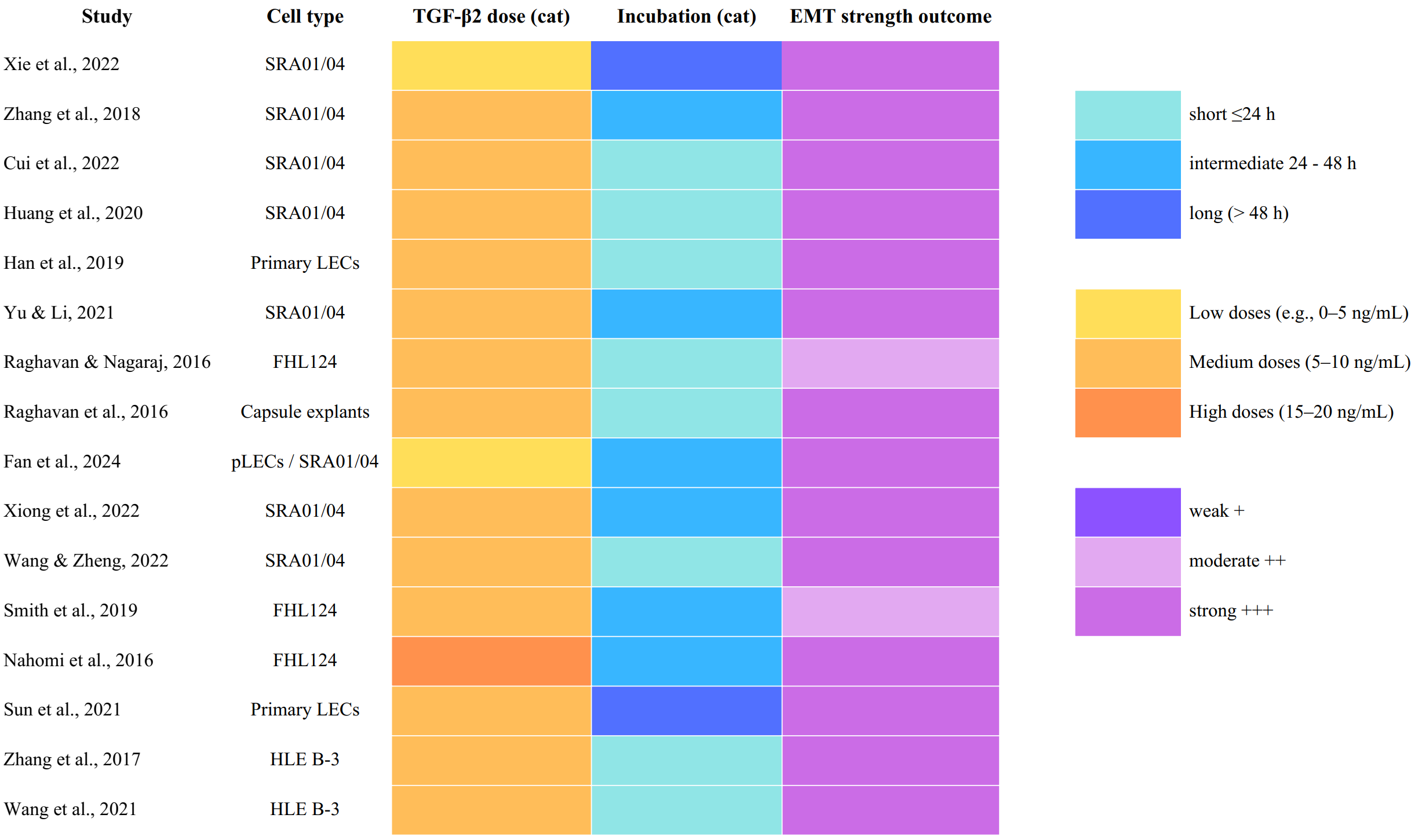
Heat-map synthesis of methodological characteristics and EMT outcomes across 16 included
DISCUSSION
Preventing posterior capsule opacification (PCO) after cataract surgery is critically important, as it remains one of the most prevalent complications, affecting approximately 50% of patients within five years postoperatively and significantly impairing visual outcomes 22. Approximately 30% of adults and 100% of children experience vision impairment due to PCO. PCO primarily results from the proliferation and migration of residual lens epithelial cells (LECs), leading to obstruction of the visual axis and often necessitating additional procedures, such as Nd:YAG laser capsulotomy 1. The persistent occurrence of this complication highlights the urgent need for effective strategies to reduce its incidence 23. Narrative reviews by Nibourg et al. (2015) 4 and Konopińska et al. (2021) 1 have comprehensively described PCO mechanisms and prevention strategies but have not offered direct comparisons of the experimental conditions used in vitro. The present review analyzes 16 studies that explore variations in culture media, TGF-β2 dosing, and incubation times, which may explain the conflicting results observed among different researchers.
Effective Dose of TGF- β2 that Induced PCO
The available studies consistently demonstrate that TGF-β2 is a potent inducer of epithelial–mesenchymal transition (EMT) in lens epithelial cells (LECs); three studies administered 5 ng/mL, whereas seven used 10 ng/mL. Importantly, a concentration of 10 ng/mL TGF-β2 induces marked morphological changes in LECs, shifting them from a single-layer, cobblestone-like morphology to elongated, spindle-shaped cells characteristic of the fibrotic remodeling that underlies posterior capsule opacification (PCO)24. Huang et al. (2020) further showed that 10 ng/mL TGF-β2 in Dulbecco’s modified Eagle’s medium (DMEM) increased SRA01/04 cell migration by approximately 2.5-fold through the miR-497-5p/circ-GGA3 axis. However, the cellular response is time-dependent; ~24 h of exposure is sufficient to trigger transcriptional changes, whereas 48–72 h is required for observable phenotypic effects (e.g., stress-fiber formation)13.
The methodologies used to evaluate the impact of TGF-β2 comprised quantitative reverse-transcription PCR (qRT-PCR) and Western blot analyses, which confirmed the up-regulation of key epithelial–mesenchymal transition (EMT) and fibrotic markers, including α-SMA, fibronectin (FN), collagen type I (Col I), vimentin and N-cadherin, together with a concomitant down-regulation of E-cadherin 24,25. These markers are critical to validating the fibrotic cataract model because they mirror the conversion of lens epithelial cells (LECs) into a myofibroblastic phenotype, a hallmark of posterior capsular opacification (PCO) 26. Notably, NEAT1 expression was also elevated in TGF-β2-treated cells, implicating this long non-coding RNA in facilitating EMT. NEAT1 participates in diverse cellular processes—including proliferation, migration and differentiation—all of which are pertinent to the fibrotic changes observed in PCO 19. Beyond morphological and molecular alterations, functional read-outs such as Transwell assays demonstrated that TGF-β2 treatment increased the viability and migratory capacity of SRA01/04 cells, thereby further supporting its role in driving EMT and PCO 12. The crosstalk of multiple signalling cascades, particularly the TGF-β/Smad and Notch pathways, has been implicated in these events, underscoring the complexity of EMT regulation in LECs 3. Collectively, these findings highlight the pivotal role of TGF-β2 in initiating EMT and promoting the development of PCO.
The Preferred Medium
Most studies utilize Dulbecco's Modified Eagle Medium (DMEM) or Minimum Essential Medium (MEM) as the basal medium 27. DMEM is preferred because its high glucose concentration (4.5 g L⁻¹), along with abundant amino acids and vitamins, supports robust cell proliferation 30. Xie et al. (2022) and Zhang et al. (2018) cultured SRA01/04 cells in DMEM supplemented with 10 % fetal bovine serum (FBS), achieving stable growth and reproducible responses to TGF-β₂ stimulation 3,7. Conversely, serum-free formulations have been adopted to minimise the influence of endogenous growth factors; for example, Fan et al. (2024) employed such media during ferroptosis assays 21. Although these findings are noteworthy, they remain preliminary and were therefore excluded from the core model-optimisation synthesis.
Fetal bovine serum (FBS) concentrations typically range from 1 % to 20 %, contingent on the experimental objective. Lower concentrations (1–5 %) preserve the slowly proliferating central-lens epithelial phenotype, whereas higher concentrations (10–20 %) approximate the proliferative zone of the lens. Wang et al. (2021) reported that supplementation with 10 % FBS in DMEM/F-12 optimally maintained HLE-B3 viability, although TGF-β2 concentrations exceeding 10 ng/mL were cytotoxic17. In contrast, serum-free protocols (e.g., Fan et al.) enable the investigation of isolated signaling pathways without interference from serum-derived growth factors21.
The choice of medium depends on the research objective. For proliferation and viability studies, a basal medium consisting of DMEM supplemented with 10 % FBS is commonly used 28,29. To enhance proliferation, epidermal growth factor (EGF, 10 ng/mL) or basic fibroblast growth factor (bFGF, 5 ng/mL) may be added 29. Additionally, an extracellular matrix (ECM) component such as gelatin or type IV collagen is frequently included to promote optimal cell adhesion 28. In contrast, for EMT and fibrosis induction, a low-serum medium (DMEM + 1 % FBS) is generally preferred to minimize serum interference 30. TGF-β2 (10 ng/mL) is routinely applied for 24–48 h to induce EMT and fibrosis 31, whereas lapatinib (1 μM) can be employed to inhibit ErbB signalling, if required 32.
Duration of TGF-β2 Intervention that Induced PCO
Incubation times following TGF-β2 treatment have been reported to range from 24 h to several days, with eight studies specifically employing a 24-h period. This interval is commonly selected because it permits the initial induction of EMT while limiting prolonged exposure that could elicit additional, potentially confounding, alterations in cellular behaviour 33. Standardizing these parameters ensures that the observed effects can be attributed to TGF-β2 per se, rather than to secondary changes induced by extended exposure 24,32.
Epithelial identity begins to deteriorate; the mRNA levels of E-cadherin and ZO-1 decrease by 50–70 % within 6–12 h, weakening intercellular junctions and loosening their tight architecture 31. Concomitantly, fibrotic features emerge, with α-SMA and fibronectin expression increasing 2–4-fold by 12–24 h, driven by Smad2/3 phosphorylation and ERK/MAPK activation 10. This transition also triggers autophagy, evidenced by LC3-II accumulation and p62 degradation, which marks a shift in cellular metabolism 33. Supporting these observations, Huang et al. (2020) demonstrated that exposure of SRA01/04 cells to TGF-β2 (10 ng/mL) for 24 h produced a 3.5-fold increase in α-SMA protein and a 70 % rise in cell migration, even though the cells had not yet undergone complete morphologic transformation 13. These findings suggest that autophagy may function as a regulatory mechanism alongside EMT, although its role appears to be secondary to the optimization of culture conditions.
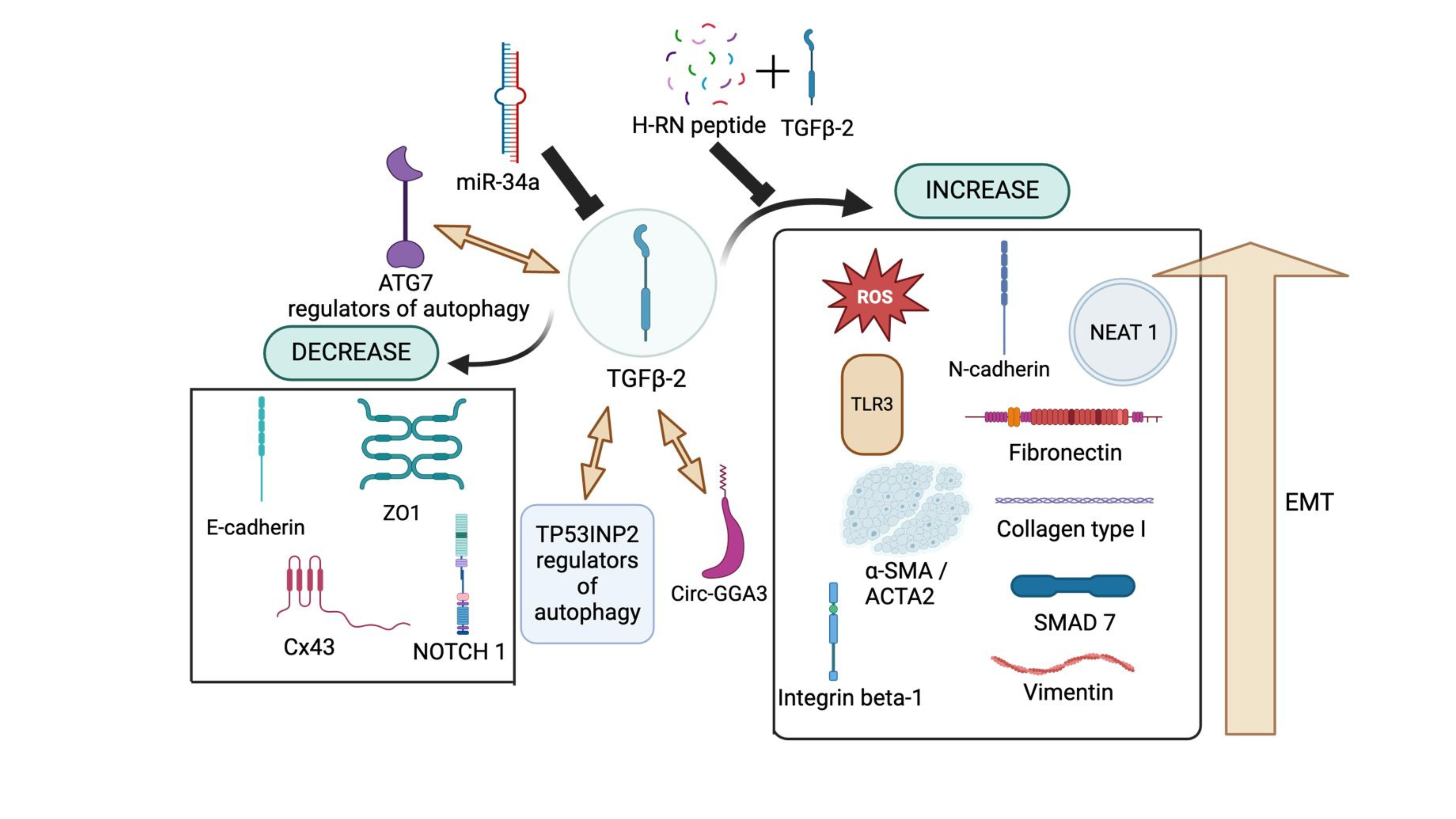
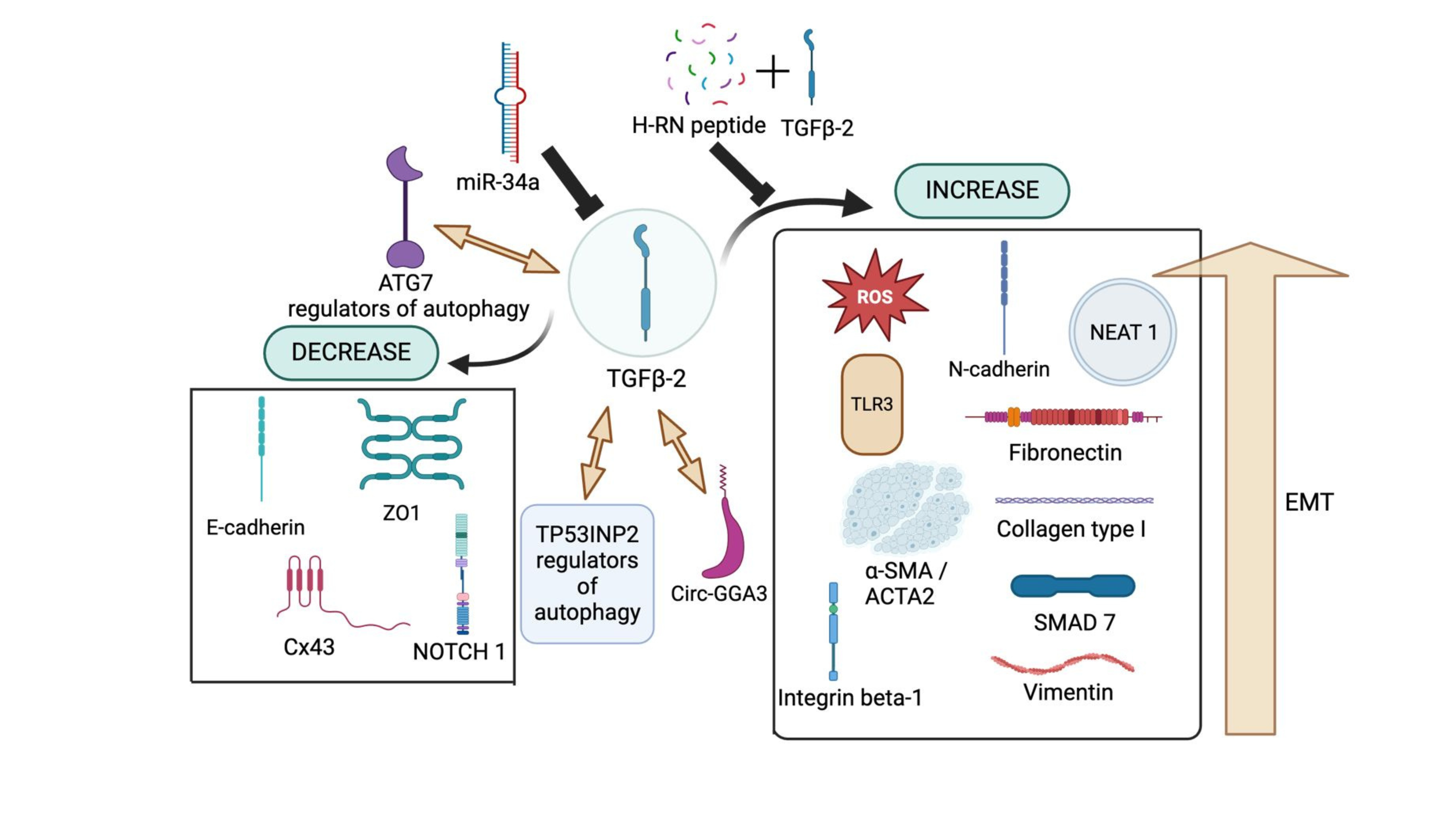
TGF-β2 Regulation on Other Proteins and RNAs. TGF-β2 upregulates or relies on circ-GGA3 to drive cellular changes associated with PCO development. Additionally, TP53INP2 and ATG7 play a crucial role in autophagy while also promoting the EMT process, further contributing to disease progression. On the other hand, H-RN peptide, when combined with TGF-β2, has been shown to inhibit EMT progression, thereby mitigating TGF-β2-driven fibrosis and EMT-related pathologies. Several studies have reported that TGF-β2 upregulates reactive oxygen species (ROS), N-cadherin (N-cad), NEAT-1, TLR3, α-SMA (ACTA2), integrin β-1, collagen type I, vimentin, and the SMAD signaling pathway, all of which are involved in the proliferation and migration of lens epithelial cells (LECs). Conversely, TGF-β2 downregulates key epithelial markers such as E-cadherin (E-cad), ZO-1, connexin 43 (Cx43), and NOTCH1, further facilitating EMT and fibrotic progression.
This review was conceived to highlight the practical considerations involved in establishing robust in vitro posterior capsular opacification (PCO) models. Although epithelial-to-mesenchymal transition (EMT) constitutes the biological foundation of these systems, experimental reproducibility is primarily governed by the technical parameters of the culture milieu. Three variables are particularly influential. First, TGF-β2 concentration: lower concentrations (0–5 ng/mL) induce EMT progressively and preserve cellular viability, whereas higher concentrations (≥10 ng/mL) provoke a rapid, fibrotic-like phenotype. Second, incubation duration: a 24 h exposure is most frequently employed and is generally sufficient to up-regulate canonical EMT markers; nevertheless, several studies have extended incubation to 48–96 h to capture more persistent responses or to evaluate therapeutic interventions. Third, the cellular source and substratum: primary human lens epithelial cells seeded on native capsule explants recapitulate the environment more faithfully than immortalised cell lines cultured on plastic. Collectively, meticulous optimisation of dose, timing and culture architecture enhances the fidelity of PCO models and facilitates inter-laboratory comparability. Unlike previous narrative reviews that considered PCO models within broader biological or clinical frameworks, the present review systematically defines the critical experimental parameters and offers actionable guidance for laboratories aiming to optimise and standardise in vitro PCO assays.
Several limitations should be acknowledged. The included studies exhibited substantial variability in cell models, culture conditions, TGF-β2 dosing, incubation times, and EMT markers, which limited comparability and precluded quantitative analysis. The vote-counting and semi-quantitative scoring approaches remain subjective, as differences in reporting detail and assay methods may affect how EMT strength is classified. Although the QUIN tool provides a structured framework, it was not designed for ophthalmic in-vitro research and may not fully capture relevant sources of bias, potentially contributing to the clustering of studies in the “low-risk” category. In addition, the review included only English-language full-text articles, which may have introduced language bias and excluded potentially relevant studies. Nevertheless, the review provides a coherent synthesis of TGF-β2-induced EMT models and highlights consistent patterns that may guide future experimental work.
CONCLUSION
The choice of culture medium for lens epithelial cells (LECs) should be guided by specific experimental objectives, the particular cell line, and its responsiveness to growth factors. Based on current evidence, our review indicates that Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (FBS) and 10 ng/mL transforming growth factor-β2 (TGF-β2) for 24–48 hours is the most consistently effective regimen for inducing epithelial–mesenchymal transition (EMT) in LECs, and therefore represents a practical in vitro recommendation for future posterior capsular opacification (PCO) investigations.
Abbreviations
AGEs: Advanced glycation end products; ARC: Age-related cataract; ATG7: Autophagy related 7; BME / BM: Basement membrane extract / Basement membrane; BPs / MFs / CCs: Biological processes / Molecular functions / Cellular components; bFGF: Basic fibroblast growth factor; Col I: Collagen type I; CTGF: Connective tissue growth factor; Cx43: Connexin 43; DEGs: Differentially expressed genes; DMEM / EMEM / MEM: Dulbecco's Modified Eagle Medium / Eagle's Minimum Essential Medium / Minimum Essential Medium; E-Cad: E-Cadherin; ECM: Extracellular-matrix; EDU: 5-Ethynyl-2'-deoxyuridine; EGF: Epidermal growth factor; EMT: Epithelial-mesenchymal transition; ERK: Extracellular signal-regulated kinases; FBS: Fetal bovine serum; FHL124: Fetal human lens epithelial-124; FN: Fibronectin; GO: Gene Ontology; HLE: Human lens epithelial; H-RN: H-RN peptide; IF: Immunofluorescence; IL-1β: Interleukin 1β; IOL: Intraocular lens; IP: Immunoprecipitation; LECs / HLECs: Lens epithelial cells / Human lens epithelial cells; MAPK: Mitogen-activated protein kinases; MeSH: Medical Subject Headings; N-cad: N-cadherin; NEAT1: Nuclear Paraspeckle Assembly Transcript 1; OSF: Open Science Framework; PCO: Posterior capsule opacification; PICO: Population, Intervention, Comparison, and Outcome; pLECs: Primary lens epithelial cells; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; qRT-PCR / qPCR: Quantitative real-time polymerase chain reaction; RAGE: Receptor for advanced glycation end products; RNA-seq: RNA sequencing; ROS: Reactive oxygen species; SOP: Standard operating procedure; TEM: Transmission electron microscopy; TGFβ / TGFβ2: Transforming growth factor beta / beta 2; TLR3: Toll-like receptor 3; TNF-α: Tumor necrosis factor alpha; TP53INP2: Tumor protein p53 inducible nuclear protein 2; VIM: Vimentin; WB: Western blot; YAG: Yttrium aluminium garnet (laser capsulotomy); ZO-1: Zonula occludens-1; α-SMA: α-Smooth muscle actin
Acknowledgments
None.
Author’s contributions
L.R.W and I.K conceptualized the study and designed the methodology, I.K performed the data collection, L.R.W and I.K performed the data analysis and wrote the main manuscript text, S.W, H.S, and H.K.S review and editing the final manuscript, S.W, H.S, and H.K.S checked for intellectual content and editing. L.R.W, M.R, R.H, and M.F.J supervised the project. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, etc) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

