
Short-term clinical outcomes in patients with acute myocardial infarction after successful percutaneous coronary revascularization: the role of promoter polymorphism of the endothelial nitric oxide synthase gene
- Department of prevention and treatment of emergency conditions, Government Institution “L.T.Malaya Therapy National Institute NAMSU”, 2A Liubovi Maloy av., 61039, Kharkiv, Ukraine
- Department of clinical pharmacology and pharmacogenetic on non-communicable diseases, Government Institution “L.T.Malaya Therapy National Institute NAMSU”, 2A Liubovi Maloy av., 61039, Kharkiv, Ukraine
- Therapeutic Unit, Internal Medicine Department, State Medical University of Zaporozhye, 26, Mayakovsky av., Zaporozhye, 69035, Ukraine
Abstract
Background: The purpose of this study was to investigate the associations between variants of a promoter polymorphism of the endothelial nitric oxide synthase (eNOS) gene and clinical outcomes in acute ST-segment elevation myocardial infarction (STEMI) patients after a successful primary percutaneous coronary artery intervention (PCI).
Methods: 177 patients with acute STEMI, 82 patients with angiographically proven stable coronary artery disease, and healthy volunteers were included in the study. The primary end-point was a combined event (follow-up major adverse cardiac events –MACEs and hospitalization) that occurred within 6-month of the discharge from the hospital.
Results: The combined end-point was determined in 72 patients from the entire acute STEMI population (40.6%), including 24 events for 786TT genotype, 23 events for 786TC genotype and 25 events for 786CC genotype. Kaplan-Meier curves demonstrated that acute STEMI patients with 786CC eNOs genotype had lower MACEs free accumulation when compared to those with 786TC and 786TT eNOs genotypes at 6-month follow up period (Log-rank p < 0.001). Multivariate Cox regression analyses identified 786CC in eNOs gene as an independent predictor of clinical outcomes in STEMI after PCI.
Conclusions: the 786CC polymorphism in eNOs gene is an independent predictor for clinical outcomes after a successful primary PCI in acute STEMI.
Introduction
Nitric oxide (NO) is an established factor in maintaining endothelial function and vascular wall integrity1,2,3,4. Genetic polymorphisms of endothelial NO synthase (eNOs) is associated with altered NO levels in peripheral blood, increased levels of plasma low-density lipoproteins (LDL) and oxidized lipids, suppressed the production of vascular endothelial growth factor and increased fasting glucose5,6,7 These findings were reported to have clinical significance in acute ST-segment elevation myocardial infarction (STEMI), stable coronary artery disease (CAD), asymptomatic atherosclerosis, heart failure (HF), abdominal obesity, hypertension, diabetes mellitus, and restenosis and thrombosis after primary percutaneous coronary artery intervention (PCI)8,9,10 Previous studies have shown that polymorphism in promoter region of eNOs gene (T786C) in STEMI patients is corresponding with impaired pleiotropic effect of NO, which led to adverse cardiac remodeling, early stent thrombosis and restenosis after PCI 11,12,13,14. T786C eNOs genotype can predict cardiovascular (CV) mortality in high-risk patients15 and individuals with stable CAD16, while several studies have reported controversies issues regarding the discriminative values of T786C eNOs genotype in STEMI patients17,18. There is limited evidence regarding the association of T786C polymorphism in eNOs gene and short-term clinical outcomes in STEMI after completed revascularization19. The study aimed to investigate the associations between SNP Т786С in eNOS gene and clinical outcomes in acute STEMI patients after a successful PCI.
Methods
Patients’ population
A total of 268 patients with confirmed acute STEMI and 82 patients with angiographically proven stable CAD, age- and sex-matched to STEMI individuals, were checked for eligibility to participate in the study (Figure 1). Fifty-five healthy volunteers were included as a healthy control group. From the entire patient population of established acute STEMI (n=268), 177 individuals, who were admitted to intensive care unit of GI “L.T.Malaya TNI NAMSU” within a given period from 2016 August to 2018 July and met inclusion/non-inclusion criteria, were enrolled in the study. Ninety-one patients with acute STEMI were excluded from the study due to incompletion of inclusion/exclusion criteria, including time window frame for performing coronary revascularization procedure. Acute STEMI was diagnosed according to the European Cardiology Society (ECS) Guidelines (2017)20. Inclusion criteria were established for acute STEMI cases of age > 18, who have no contraindications to PCI. Cases with previous myocardial infarction, established chronic heart failure, known malignancy, severe comorbidities (anemia, chronic obstructive lung disease, bronchial asthma, liver cirrhosis, chronic kidney disease, valvular heart disease, bleeding), inability to understand of written informed consent were excluded from the study. Primary PCI with bare-metal stent (COMMANDER, “Alvimedica”, Turkey) implantation was performed. All acute STEMI patients received adjuvant treatment due to current ESC recommendations20.
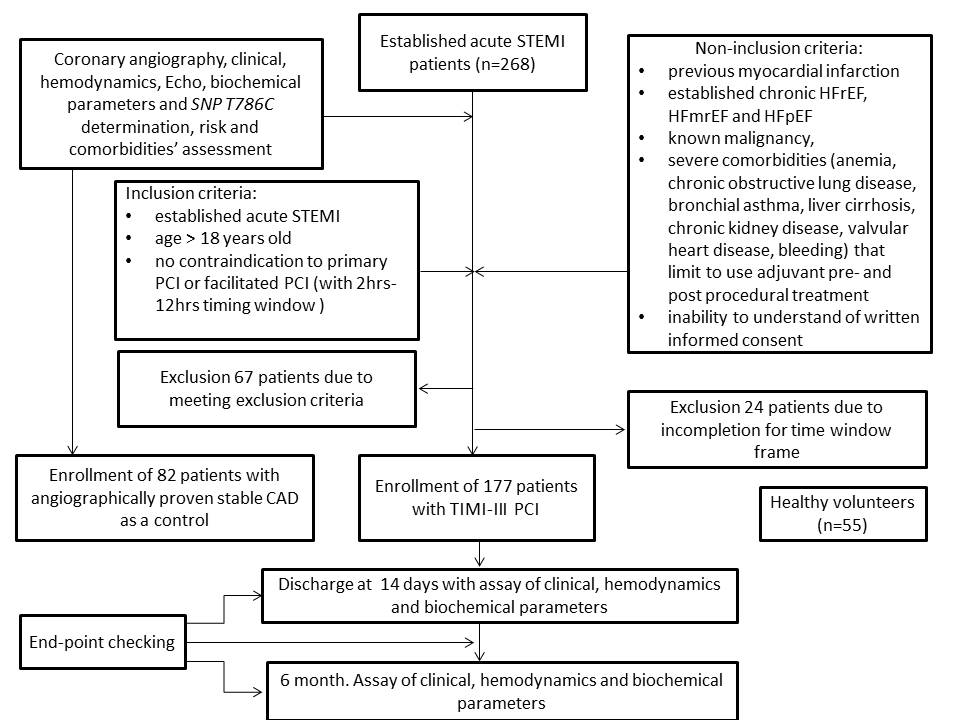
The flow chart of study design, inclusion / exclusion criteria and study procedures.
Ethical declaration
All procedures performed in this study that involved human participants were in accordance with ethical standards, the 1964 Helsinki declaration and its later amendments, or comparable ethical standards, and were approved by the local ethics committee (Protocol №8, 29.08.2016). Informed consent was obtained from each patient.
Determination of endpoints
The primary end-point was combined events (follow-up major adverse cardiac events –MACEs and hospitalization) that occurred within 6-month after the discharge from the hospital21. MACEs were defined as the composite of CV death, recurrent MI, newly diagnosed HF. CV death was confirmed by personal or phone contact to the family doctor or the hospital where the patient died. The diagnosis of myocardial infarction 20 and heart failure (HF) 22 has been established according to the ESC clinical guidelines. Hospitalization was ascertained by direct or phone contact with the hospital reception, where the patient was admitted.
Sample size calculation
The sample size was calculated through the effect size estimation (0.99), the type of present study, providing study power of 80% and type I error 5%23. The sample size was 150 individuals.
Coronary angiography
Conventional coronary angiography was performed immediately after admission of the patients to the hospital using Digital X-Ray system “Integris Allura” (Philips Healthcare, Best, The Netherlands) and managed by radial vascular access as per conventional protocol. In this study, the contrast “Ultravist-370” (Baier Pharma GmbH, Germany) and automatic contrast injector were used. The coronary arteries were divided into segments according to the American Heart Association classification24.
Determination of STEMI prognosis
TIMI score was used to validate prognostic capacity after STEMI25.
SYNTAX score determination
SYNTAX score (SS) was used to assess the severity of coronary atherosclerotic lesions and was calculated by an experienced interventional cardiologist26.
Determinationof riskfactors and comorbidities
Dyslipidemia was diagnosed according to the ECS dyslipidemia guideline (2016)27. Hypertension was diagnosed if systolic blood pressure (SBP) was >140 mm Hg, and/or diastolic blood pressure (DBP) was >90 mm Hg according to the ECS guideline on diagnostics and treatment of arterial hypertension (2018)28. Type 2 diabetes mellitus (T2DM) was determined according to the new ADA statement (2017)29.
Echo and Doppler examination
Echo-CG was performed on an “Aplio 500” (TUS-A500, TOSHIBA MEDICAL SYSTEMS corporation Japan) using a 3.5 MHz phase probe at discharge and 6-month observation period. LV end diastolic volume (EDV), LV end systolic volume (ESV), and LV EF measurement was performed according to Simpson's biplane method as per the contemporary recommendation of American Society of Echocardiography30. LV global longitudinal strain (e`) and early transmitral velocity (E) were measured by tissue Doppler imaging technique and impulse transmitral Doppler regime at baseline and 6-month follow-up. Left ventricular myocardial mass (LVMM) was calculated automatically based on the protocol of echocardiograms evaluation31.
Blood samples
Blood samples were collected immediately before PCI and at six months of investigation. Blood samples were centrifuged, and serum was isolated within 30 minutes after sample collection. Then, final samples were frozen at -70C and stored in plastic tubes until further examination.
Troponin I (Tn I) level was measured by chemo-luminescent immunoassay (Humalyser 2000, Mannheim, Germany). The TnI level average was 0.5-50 ng/mL.
Total cholesterol (TC), low LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride (TG) levels were measured by a direct enzymatic method (Roche P800 analyzer, Basel, Switzerland). The intra-assay and inter-assay coefficient variation was <5%.
Fasting glucose levels were measured by a double-antibody sandwich immunoassay (Elecsys 1010 analyzer, F. Hoffmann-La Roche Diagnostics, Mannheim, Germany). The intra-assay and inter-assay coefficient variation was <5%.
Total creatine kinase (CK) and CK MB-fraction (CK-MB) levels were analyzed using immunoinhibition method on a quantitative immunoassay analyzer Humalyser 2000 (HUMAN GmbH, Germany), according to the manufacturers’ recommendations
N-terminal fragments of brain natriuretic peptide (NT-proBNP) were measured by commercially available standard kit (R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany). The NT-proBNP level average was 10-12000 pg/mL.
Plasma stable metabolic end products of NO
The blood sample for NO measure was collected before nitroglycerin was given through . or orally. The stable metabolic end products of NO (NOx=NO2 plus NO3) in the supernatants of peripheral blood samples were analyzed by Griess method28. We performed measurement using the commercial “Total Nitric Oxide and Nitrate/Nitrite Parameter Assay” Kit produced by RnD Systems (Minneapolis, MN, USA) and analyzer Immunochem-2100 (High technology, Inc., USA). The level of average was 0.78–200 µmol /L. Analytical sensitivity was 0.78–200 µmol/L.
SNP T786C (rs2070744) in eNOS gene determination
The DNA extraction was performed according to the protocol of a commercial set, DNA-Sorb-B (AmplySens, Russia). The assessment of allelic states of SNP studied was performed using real-time (RT) polymerase chain reaction (PCR). The commercial qPCR mix kit, SNP-Screen (catalog number NP-554-100, Syntol, Russia), was used for RT-PCR.
primer sequences were as follows:
F: 5’-ACCAGGGCATCAAGCTCTTC-3’,
: 5’-GCAGGTCAGCAGAGAGACTAG-3’,
rs2070744-C: 5’-VIC-AGGGTCAGCCGGCCAG-BHQ1-3’, C
rs2070744-T: 5’-FAM-AGGGTCAGCCAGCCAG-BHQ1-3’. T
PCR was performed on a CFX96 thermocycler (BioRad, USA) using an allelic discrimination test. PCR conditions: 95°C – 3', 40 cycles of 95°C – 15'', 65°C – 40''
Statistics
Statistical analyses were performed using SPSS for Windows v.23 (USA). Continuous variables are presented as mean ± standard deviation (SD) when normally distributed, or median and interquartile range (IQR) if otherwise. Categorical variables are presented as frequencies (n) and percentages (%). Differences between groups were assessed using the unpaired two-tailed Student’s t-test or Mann-Whitney U test and the chi-square test for continuous and categorical variables, respectively. Allele frequencies were estimated, and all genetic polymorphisms of eNOs were tested for Hardy–Weinberg Equilibrium. Univariate correlation and linear regression analysis (stepwise) were performed to identify independent risk factors for combined end-point in STEMI patients (MACE + hospitalization). We calculated beta coefficient, standard deviation (SD), odds ratio (OR), and 95% confidence interval (CI) for each factor. Factors, for which P values were calculated as >0.1, were not included in the multivariate log-regression. The multiple continuous dependent variables analysis (MANCOVA) was used to assess statistical differences between multiple continuous variables. Wilk’s λ and partial eta squared (η2) were calculated for each predictor. The Kaplan-Meier method and the log-rank test were used to compare the rates of combined endpoints in STEMI patients with , polymorphisms of eNOs according to 6-month survival rates. Multivariate Cox regression analyses, which were adjusted for SYNTAX score, abdominal obesity, T2DM and dyslipidemia (those variables with a significance level of p <0.05 in a univariate test and clinically considered valuable) were conducted. All differences were considered statistically significant with 2-tailed p<0.05.
Results
The observed frequency of Т786С eNOs genotypes in the population of acute STEMI patients (n=177) was as follows: TT=41.2% (n=73), ТС = 36.1% (n=64) and CC=22.6% (n=40) respectively. Deviation from Hardy–Weinberg equilibrium was noted due to an excess of homozygosity (χ2=6.31, P=0.012). The expected frequency of Т786С eNOs genotypes determined in stable CAD patients (n=82) was as follows: TT=47.6% (n=39); CT=37.8% (n=31); CC=14.6% (n=12). There was no deviation from the Hardy–Weinberg equilibrium (χ2=2.2985, P=0.129). Healthy volunteers (n=55) had TT eNOs genotype in 56.4% (n=31); CT genotype in 32.7% (n=18); and CC genotype in 10.9% (n=6) of cases without significant deviation from expecting frequencies (χ2=1.6844, P=0.194).
Characteristics of stable CAD and STEMI patients included in the study
Entire stable CAD population (n=82) | Entire STEMI population (n=177) | STEMI population (n=177) | P value | |||
TT genotype (n=73) | TC genotype (n=64) | CC genotype (n=40) | ||||
1 | 2 | 3 | ||||
| Age, years (SD) | 58.60±7.20 | 61.73±9.44 | 59.00±10.10 | 59.27±9.92 | 58.53±8.29 | |
| Male, n (%) | 63 (76.8%) | 139 (78.5%) | 57 (78.1%) | 50 (78.1%) | 32 (80.0%) | |
| Female, n (%) | 19 (23.2%) | 38 (21.5%) | 16 (21.9%) | 14 (21.9 %) | 8 (20.0%) | |
| Hypertension, n (%) | 59 (71.9%) | 146 (82.5%)# | 60 (82.2 %) | 55 (85.9 %) | 31 (77.5 %) | |
| T2DM, n (%) | 19 (23.1%) | 44 (24.9%) | 15 (20.5 %) | 13 (20.3 %) | 16 (40.0 %) | P1-3 =0.027P2-3 =0.029 |
| Smoking, n (%) | 37 (45.1%) | 84 (47.5%) | 29 (39.7 %) | 31 (48.4 %) | 24 (60.0 %) | P1-3 =0.039 |
| HCE, n (%) | 44 (53.6%) | 105 (59.3%) | 45 (61.6 %) | 37 (57.8 %) | 23 (57.5 %) | |
| BMI>30 кг/м2, n (%) | 20 (24.4%) | 69 (39.0%)# | 29 (39.7 %) | 26 (40.6 %) | 14 (35.0 %) | |
| Stable CAD prior to STEMI, n (%) | - | 107 (60.5%) | 42 (57.5%) | 39 (60.9 %) | 26 (65.0 %) | |
| Unstable angina prior to STEMI, n (%) | - | 67 (37.9%) | 21 (28.8 %) | 24 (37.5 %) | 22 (55.0 %) | P1-3 =0.011P2-3 =0.080 |
| Peak TnI, ng/mL | - | 17.7 [6.34-77.2] | 17.97 [9.73-60.5] | 17.7 [3.87-129.0] | 25.05 [3.99-180.0] | |
| Peak CK-MB,U/L | - | 103.3 [44.9-28.95] | 108.90 [74.30-328.40] | 112.90 [55.00-196.90] | 43.85 [30.20-158.50] | |
| NT-proBNP at admission, pg/mL | 96 [86 - 118] | 246 [107 - 405]# | 247 [118 - 388] | 253 [121 - 375] | 241 [105 - 344] | |
| NT-proBNP at discharge, pg/mL | 89 [82 - 105] | 121 [88 - 145] | 117 [92 - 144] | 119 [90 - 147] | 123 [93 - 151] | |
| Total cholesterol, mmol / L | 4.91 [4.12-6.20] | 4.82 [3.95-5.63] | 4.83 [3.91-5.79] | 4.62 [3.95-5.50] | 4.89 [3.79-5.58] | |
| HDL-cholesterol, mmol / L | 1.06 [0.94-1.17] | 1.12 [0.92-1.28] | 1.12 [0.92-1.28] | 1.13 [0.94-1.31] | 1.05 [0.90-1.28] | |
| LDL cholesterol, mmol / L | 3.14 [2.20-4.10] | 3.00 [2.03-3.63] | 2.64 [1.87-3.46] | 2.79 [2.04-3.56] | 3.36 [2.46-4.13] | P1-3 =0.048 |
| NO(x), micromole/L | 17.10 [12.50 – 21.40] | 25.90 [14.76 – 40.71]# | 39.55 [21.46 – 50.40] | 31.67 [20.46 – 43.74] | 16.43 [10.55 – 22.21] | P1-3 =0.012 |
There were significant differences between stable CAD and acute STEMI patients in frequencies of hypertension, obesity, serum levels of NT-proBNP, and NO(x) (
STEMI localization and number of damaged coronary arteries in patients included in the study depending on major / minor alleles at baseline
Entire population n=177 | TT genotype n=73 | TC genotype n=64 | CC genotype n=40 | P value | |
1 | 2 | 3 | |||
STEMI risk scoring | |||||
| TIMI risk score, point | 6 [4-7] | 6 [5-7] | 6 [4-7] | 7 [5-8] | 0.62 |
| SYNTAX score, point | 28.7±6.1 | 23.6±4.8 | 25.8±5.1 | 31.9±5.4 | 0.043 |
| >32 points, n (%) | 76 (42.9) | 20 (27.4) | 25 (39.1) | 31 (77.5) | 0.042 |
| 22 - 32 points, n (%) | 79 (44.6) | 42 (57.5) | 32 (50.0) | 5 (12.5) | 0.041 |
| ≤22 points, n (%) | 22 (12.4) | 11 (15.1) | 7 (10.9) | 4 (10.0) | 0.050 |
| STEMI Localization | |||||
| Anterior LV wall, n (%) | 97 (54.8%) | 44 (60.3 %) | 33 (51.6 %) | 20(50.0 %) | |
| Posterior LV wall, n (%) | 70 (39.5%) | 29 (39.7 %) | 31 (48.4 %) | 20 (50.0 %) | |
Amount of coronary vessels injured | |||||
| One artery, n (%) | 53 (29.9%) | 24 (32.9 %) | 18 (33.3 %) | 11 (33.3 %) | |
| Two and more arteries, n (%) | 103 (58.2%) | 45 (61.6 %) | 36 (66.6 %) | 22 (66.6 %) | |
| Left anterior descending, n (%) | 47 (26.6%) | 25 (34.2%) | 9 (14.1%) | 13 (32.5%) | P1-3 =0.009P1-3 =0.048 |
| Right coronary artery, n (%) | 41 (23.2%) | 22 (30.1%) | 12 (18.8%) | 7 (17.5%) | |
| Circumflex coronary artery, n (%) | 20 (11.3%) | 9 (12.3%) | 6 (9.4%) | 5 (12.5%) | |
| Left main, n (%) | 12 (6.8%) | 7(9.6%) | 2 (3.1%) | 3 (7.5%) | P1-3=0.035P1-3=0.05 |
There were no significant differences between the three cohorts in TIMI risk, STEMI localization, numbers of damaged coronary arteries, and complication of MI. However, damages in the left anterior descending artery were frequent in patients with CC genotype of eNOs gene (
Additionally, left primary damage was more frequent in individuals with TT genotypes than those who had TC genotype, but there was no significant difference in the frequency of the left primary damage among patients with CC, TC and TT genotypes.
Combined end-point was determined in 72 patients from entire acute STEMI population (40.6%), including 24 events occurred in 786TT in eNOs genotype, 23 events were determined in 786TC in eNOs genotype and 25 events were found in 786CC in eNOs genotype. There were no significant differences between patients’ cohorts within in-hospital periods, while heart failure, MACE, hospitalization, and combined endpoint were frequent in STEMI patients with CC genotype (
Clinical events in acute STEMI patients
Entire population n=177 | TT genotype n=73 | TC genotype n=64 | CC genotype n=40 | P value | |
1 | 2 | 3 | |||
In-hospital events in STEMI patients | |||||
| Total quantity of complicated acute STEMI, n (%) | 69 (38.9%) | 30 (41.1 %) | 22 (34.4 %) | 17 (42.5 %) | |
| II-III Killip class of HF, n (%) | 28 (15.8%) | 16 (21.9 %) | 8 (12.5 %) | 4 (10.0 %) | |
| IV Killip class of HF, n (%) | 10 (0.6%) | 5 (6.8 %) | 1 (1.6 %) | 4 (10.0 %) | |
| Cardiac arrest, n (%) | 3 (1.7%) | 1 (1.4 %) | 0 | 2 (5.0 %) | |
| Acute 2-3 grade av-block, n (%) | 5 (2.8%) | 2 (2.7 %) | 1 (1.6 %) | 2 (5.0 %) | |
| Recurrent angina, n (%) | 1 (0.6%) | 1 (1.4 %) | 0 | 0 | |
Six-month events in STEMI patients after discharge from the hospital | |||||
| Heart failure, n (%) | 46 (26.0%) | 14 (19.2%) | 17 (26.6%) | 15 (37.5%) | P1-3 =0.033 |
| CV death, n (%) | 12 (6.8%) | 5 (6.8%) | 4 (6.25%) | 3 (7.5%) | |
| MACE, n (%) | 58 (32.8%) | 19 (26.0%) | 21 (32.8%) | 18 (50.0%) | P1-3 =0.038P2-3 =0.040 |
| Hospitalization, n (%) | 17 (9.6%) | 5 (6.8%) | 5 (7.8%) | 7 (17.5%) | 1-3 =0.022 2-3 = 0.022 |
| Combined end point, n (%) | 72 (40.6%) | 24 (32.9%) | 23 (35.9%) | 25 (62.5%) | 1-3 =0.025 2-3 = 0.036 |
Hemodynamics in STEMI patients enrolled in the study
Parameters | TT genotype n=73 | TC genotype n=64 | CC genotype n=40 | P value |
At baseline | ||||
| HR, per min | 80.81±16,68 | 74.17±14.84 | 77.00±16.64 | PTT-CC =0.012 |
| SBP, mmHg | 138.48±27.13 | 136.70±29.00 | 139.00±27.52 | |
| DBP, mmHg | 82.14±13.18 | 80.92±12.72 | 80.00±13.88 | |
| LV EDV, ml | 137.60±33.36 | 131.91±45.47 | 149.72±44.63 | |
| LV ESV, ml | 65.08±23.34 | 60.98±31.27 | 77.32±36.96 | |
| LA, cm | 4.14±0.60 | 4.09±0.44 | 4.22±0.43 | |
| LV EF, % | 51.25±8.23 | 54.59±8.96 | 50.84±11.96 | |
| Е/А, unit | 1.00±0.53 | 1.03±0.46 | 1.16±0.52 | |
| E/e`, unit | 8.44±1.27 | 9.18±1.25 | 13.90±1.90 | PTT-CC =0.026PCT-CC =0.046 |
| LV MM, g | 266.39±70.88 | 249.63±74.72 | 268.98±93.31 | |
At 6 month | ||||
| HR, per min | 68.95±10.03 | 69.78±12.51 | 68.83±12.18 | |
| SBP, mmHg | 140.00±15.33 | 131.11±15.37 | 132.29±14.14 | |
| DBP, mmHg | 83.33±10.29 | 85.56±16.85 | 83.13±10.30 | |
| LV EDV, ml | 140.97±35.84 | 144.42±48.25 | 159.26±56.88 | |
| LV ESV, ml | 65.54±24.18 | 68.07±31.29 | 82.53±48.66 | |
| LA, cm | 4.14±0.51 | 4.13±0.59 | 4.35±0.66 | |
| LV EF, % | 53.89±8.95 | 53.21±8.80 | 50.75±11.55 | |
| Е/А, unit | 1.20±0.56 | 1.14±0.56 | 1.21±0.68 | |
| E/e`, unit | 9.15±1.33 | 11.25±1.21 | 18.32±2.44 | PTT-CC =0.014PcT-CC =0.042 |
| LV MM, g | 242.30±89.12 | 230.59±71.48 | 265.24±76.45 | |
Hemodynamics in STEMI patients enrolled in the study is reported in
There were positive correlations between СС genotype and the combined end point (r=0.54; P=0.0001), SYNTAX score (r=0.34; P=0.002), LDL cholesterol (r=0.32; P=0.012), T2DM (r=0.30; P=0.042), abdominal obesity (r=0.28; P=0.016), TIMI score (r=0.26; P=0.012), unstable angina prior to acute STEMI (r=0.25; P=0.047), E/e` ration (r=0.23; P=0.048) and total quantity of complications due to acute STEMI within admission to the hospital (r=0.23; P=0.042). CC genotype was inversely correlated with NO(x) (r=-0.52; P=0.001), LV EF (r=-0.33; P=0.001) and diastolic BP (r=-0.26; P=0.048) in acute STEMI patients at baseline. There were no significant associations between 786СС eNOs gene polymorphism and acute STEMI localization, the levels of circulating biomarkers of acute myocardial injury/necrosis (peak levels of TnI and CK-MB), NT-proBNP at discharge, and the numbers of damaged coronary arteries.
The factors contributing 6-month combined end point after STEMI: The results of univariateand multivariate linear regressions
Data | Depending variable: combined end point | |||||||
Univariate linear regressive analysis | Multivariate linear regressive analysis | |||||||
β-coefficient | OR | 95% CІ | Р | β-coefficient | OR | 95% CІ | Р | |
| 786СС genotype of eNOS | 1.58366 | 4.8728 | 1.4093 – 16.8481 | 0.0123 | 1.57342 | 4.8231 | 1.5349 – 15.1552 | 0.0071 |
| SYNTAX score | 1.17560 | 1.9428 | 1.2493 – 3.5422 | 0.0244 | 1.41380 | 1.6844 | 1.183 – 2.3655 | 0.0234 |
| TIMI score | 1.37250 | 1.8970 | 0.9720 – 2.880 | 0.0410 | 1.17280 | 1.0940 | 1.010 – 1.3240 | 0.0520 |
| Smoking | 0.51264 | 1.6697 | 0.3756 – 7.4222 | 0.5006 | - | - | - | - |
| T2DM | 0.35065 | 0.7042 | 0.2961 – 1.6748 | 0.4276 | - | - | - | - |
| Abdominal obesity | 1.12320 | 2.1448 | 0.4607 – 3.8995 | 0.0383 | 1.02 | 1.9560 | 0.0774 – 3.4539 | 0.0526 |
| II-III Killip class of acute HF before PCI | 0.14908 | 0.8615 | 0.0713 – 4.3338 | 0.8565 | - | - | - | - |
| Stable CAD prior to STEMI | 0.43968 | 1.5522 | 0.3988 – 6.0419 | 0.5260 | - | - | - | - |
| Unstable angina prior to acute STEMI | 0.78264 | 2.3177 | 1.0611 – 4.1522 | 0.0462 | 0.71551 | 1.2317 | 0.9815 – 4.1772 | 0.1622 |
| Multiple coronary vessel injury | 0.22359 | 0.7996 | 0.1766 – 1.2622 | 0.3370 | - | - | - | - |
| E/e` at discharge | 0.35360 | 0.9160 | 1.0136 – 1.1630 | 0.0870 | - | - | - | - |
| LDL cholesterol | 0.72550 | 1.4271 | 0.9388 – 3.229 | 0.6630 | - | - | - | - |
| NT-proBNP at discharge | 1.18440 | 1.7044 | 1.0633 – 2.954 | 0.03420 | 1.17230 | 1.0144 | 1.0330 – 1.1422 | 0.0620 |
| Peak TnI at admission | 0.97510 | 1.1774 | 1.0814 – 1.302 | 0.0467 | 0.9880 | 1.1034 | 1.0024 – 1.1852 | 0.0710 |
| Peak CK-MB at admission | 0.47640 | 1.0254 | 1.0180 – 1.104 | 0.4820 | - | - | - | - |
| Dyslipidemia | 0.4582 | 0.8848 | 0.6638 – 1.1255 | 0.6388 | - | - | - | - |
The comparison of statistical difference between predictors for combined end point: The results of the multivariate analysis ofcovariance (MANCOVA)
Predictors | Wilk’s λ | F | Partial η2 | Observed power |
| Intercept | 0.77 | 9.232 | 0.277 | 1.000 |
| 786СС genotype of eNOS | 0.81 | 12,44* | 0.342 | 1.000 |
| SYNTAX score > 32 units | 0.62 | 9.65* | 0.311 | 0.946 |
| TIMI score > 7 units | 0.57 | 6.57* | 0.295 | 0,766 |
| E/e`>18 unit | 0.43 | 3.22 | 0,23 | 0.288 |
| Abdominal obesity | 0.53 | 6.34 | 0.58 | 0.477 |
| Unstable angina prior to acute STEMI | 0.59 | 6.11 | 0.73 | 0.512 |
| NT-proBNP at discharge | 0.68 | 4.25 | 0.072 | 0.299 |
| Peak TnI at admission | 0.77 | 4.07 | 0.068 | 0.316 |
| Dyslipidemia | 0.61 | 5.38 | 0.24 | 0.246 |
Determination of predictors of 6-month end-point
The univariate linear regression (stepwise) analysis has allowed verifying 786СС eNOs genotype, SYNTAX score, TIMI score, abdominal obesity, NT-proBNP and peak TnI at admission and unstable angina before acute STEMI as predictors for meeting combined end-point (
Multivariate analysis of covariance showed that 786СС genotype of eNOS (Wilk’s λ = 0.81; F=12.44; Partial η2 = 0.342), SYNTAX score > 32 units (Wilk’s λ = 0.62; F=9.65; Partial η2 = 0.311) and TIMI score > 7 units (Wilk’s λ = 0.57; F=6.57; Partial η2 = 0.295) had independent power on dependent variables entitled combined end-point (MACE + hospitalization) (
Predictors for end point accumulation: Results of Unadjusted and Adjusted Cox regression for SYNTAX score, TIMI score and T2DM
Predictors | Unadjusted Cox regression | Adjusted Cox regression | ||||
OR | 95% CI | P value | OR | 95% CI | P value | |
| 786СС eNOs genotype | 1.46 | 1.16 – 1.89 | 0.003 | 1.48 | 1.12 – 1.90 | 0.002 |
| SYNTAX score >33 units | 1.26 | 1.13 – 1.44 | 0.001 | - | - | - |
| TIMI score>7 units | 1.15 | 1.08 – 1.28 | 0.001 | - | - | - |
| Abdominal obesity | 1.04 | 1.00 – 1.10 | 0.460 | 1.02 | 1.00 – 1.05 | 0.788 |
| T2DM | 1.18 | 1.04 – 1.28 | 0.002 | - | - | - |
| E/e`>18 unit | 1.03 | 1.00 – 1.07 | 0.054 | 1.02 | 1.00 – 1.05 | 0.784 |
| Unstable angina prior to acute STEMI | 1.07 | 1.01 – 1.13 | 0.048 | 1.00 | 0.98 – 1.04 | 0.908 |
| NT-proBNP at discharge | 1.04 | 1.02 – 1.09 | 0.046 | 1.03 | 1.00 – 1.07 | 0.606 |
| Peak TnI at admission | 1.02 | 1.00 – 1.05 | 0.426 | 1.02 | 1.00 – 1.04 | 0.776 |
| Dyslipidemia | 1.01 | 0.93 – 1.11 | 0.760 | 1.00 | 0.96 – 1.06 | 0.790 |
Kaplan-Meier analysis for end-point accumulation trends in STEMI patients with eNOs genotypes
Kaplan-Meier curves demonstrated that acute STEMI patients with 786СС eNOs genotype had a lower MACEs free accumulation when compared to those with 786TC and 786TT eNOs genotypes at six months follow up period (Log-rank p < 0.001) (Figure 2). There was no significant difference between 786TC and 786TT eNOs genotypes in accumulation of combined clinical end-point.

Kaplan-Meier analysis for end point accumulation trends in STEMI patients with different eNOsgenotypes.
Multivariate Cox regression analyses
Multivariate Cox regression analyses, which were adjusted for SYNTAX score, TIMI score, T2DM showed that 786СС eNOs genotype has remained a powerful independent predictor of accumulation of combined end-point in acute STEMI patients after a successful primary PCI for a 6-month out-hospital period (
Discussion
The results of our study have demonstrated significant associations between 786CC eNOS polymorphism and short-term clinical outcomes in acute STEMI patients after a successful primary PCI. This is new evidence regarding the discriminative values of 786CC eNOs genotype in accumulation of clinical events, after adjustment for SYNTAX score, TIMI score, and T2DM. Dyslipidemia and lipids in peripheral blood were not the factors contributing to the impact of 786CC eNOS polymorphism on clinical outcomes, even though T2DM was determined as a predictor. Previous clinical studies have shown that several comorbidities (T2DM, dyslipidemia, the severity of atherosclerosis, smoking) were reported as co-factors influenced on natural evolution of acute myocardial infarction13,32. Early investigations were not confirmed as predictive potency of 786СС in eNOs gene polymorphism in acute STEMI patients17,18,as these studies were performed before the primary PCI era. We suggest that the relationship of eNOS gene variants with short-term clinical outcomes in acute STEMI patients could be clinically significant in TIMI III revascularization after primary PCI, but we could not compare the effect of 786СС polymorphism in eNOs gene in TIMI I-II STEMI individuals due to ethical reasons. Because altered NO bioavailability in patients with 786СС polymorphism in eNOs gene can cause endothelial cell dysfunction, restenosis and early thrombosis of the stent33,34, we received a clinical confirmation regarding the independent negative impact of 786СС polymorphism in eNOs gene on MACE and hospital admission after STEMI over 6 months after completed reperfusion. Additionally, this finding can be important for acute STEMI patients with angiographically normal large coronary arteries, because there was evidence that 786СС polymorphism was associated with acute myocardial infarction, especially without severe coronary organic stenosis35,36,37. However, a risk of STEMI-related complications can sufficiently increase in individuals with 786CC polymorphism38,39. Possible molecular mechanisms contributing to unfavorable clinical evolution after TIMI III reperfusion in presenters of 786CC polymorphism in eNOs gene can be restenosis, thrombosis, and heart failure developing due to adverse cardiac remodeling and no-reflow phenomenon. Indeed, we found that diastolic function in patients with 786TC / 786TT polymorphism was better than in individuals with 786CC polymorphism, but global function assayed with LVEF calculation did not differ. Probably, pre-existing severity of atherosclerosis, T2DM, and other metabolic disorders, such as dyslipidemia, influenced the restoring of blood flow after PCI and determined the support of cardiac structure and function. In fact, eNOs gene sequence variations have been reported as crucial factors for CV risk40,41, and in close relation to conventional CV risk factors42,43. Promoter polymorphism of the eNOs gene was reported with tight association with reduced mRNA and protein expression in damaged myocardium and that this altered expression has corresponded to other CV risk factors, which led to declined levels of vasoprotective agents, such as vascular endothelial growth factor44,45. Thus, 786СС polymorphism in eNOs gene accompanied CV risk factors could be used to determine abnormal myocardial perfusion after completed revascularization in acute STEMI patients. Consequently, it is suggested that impaired cardiac function due to microvascular blood flow abnormality could be the main cause that leads to worsening of clinical outcomes over six months after TIMI III reperfusion with PCI in acute STEMI patients with 786СС polymorphism in eNOs gene. The future investigation could be directed to identify the predictive values of 786СС polymorphism in eNOs gene in a larger population of acute STEMI patients with different approaches to reperfusion and various successes in PCI-related blood flow restoring.
Conclusions
In conclusion,786СС polymorphism in eNOs gene may be an independent predictor for MACE and recurrent hospital admission after successful primary PCI in acute STEMI patients.
List of abbreviations
ADA: American Diabetic Association
CAD: coronary artery disease
CI: 95% confidence interval
CK-MB: creatinine kinase isoenzyme-MB
CV: cardiovascular
DBP: diastolic blood pressure
E: early transmitral velocity
e`: global longitudinal LV strain
ECS: European Cardiology Society
EDV: end diastolic volume
EF: ejection fraction
eNOS: endothelial NO synthase
ESV: end systolic volume
HDL: high density lipoprotein
HF: heart failure
IQR: interquartile range
LDL: low density lipoprotein
LV: left ventricular
MACE: major cardiovascular events
MANCOVA: multiple continuous dependent variables analysis
NO: nitric oxide
NT-proBNP: NT-fragment pro-natriuretic peptide
PCI: percutaneous coronary intervention
SBP: systolic blood pressure
SD: standard deviation
STEMI: ST segment elevation myocardial infarction
T2DM: type 2 diabetes mellitus
TC: total cholesterol
TG: triglycerides
Competing Interests
There are no conflicts of interest.
Authors' Contributions
Conception and design: OVP; writing of the article OVP, MPK, and AEB; collection of clinical data: OVP, DPB and MPK; statistical analysis: OVP and AEB; critical revision of the article for intellectual content: AEB; interpretation of the data: OVP, MPK, and AEB; visualization procedures: DPB.
Funding
The study is a fragment of the research project: “To study the biochemical, genetic mechanisms of reperfusion damage of the myocardium and to assess the cardioprotective effect of antiplatelet therapy in acute myocardial infarction”, State Registration No. 0117U003028 / Ukraine.

