
Pathophysiology of diabetes mellitus complications: Metabolic events and control
- Department of Biochemistry, Imo State University, Owerri, Nigeria
- Department of Biochemistry, Federal University of Technology, Owerri, Nigeria
Abstract
Background: Diabetes mellitus (DM) is a metabolic disorder that is characterized by hyperglycemia and glucose intolerance, which is associated with impaired insulin secretion and peripheral sensitivity and eventual b-cell dysfunction. This review summarized the major metabolic pathways leading to both microvascular and macrovascular complications in DM, with a view of highlighting the enzymes involved and the possible inhibition of the enzymes facilitating these processes as a measure of diabetic control.
Methods: Data used in writing this review were sourced online from scientific search engines such as Google Scholar, Scopus, EMBASE, PubMed, ResearchGate, Mendeley, Medline, and SpringerLink, using keywords such as 'diabetic complications', 'hyperglycemia-induced diabetic mechanisms', 'diabetic enzymes' and 'diabetic enzyme inhibitors'. A total number of 109 references published online between 1990 and 2020 were generated and cited in this review.
Results: The most scourging and dilapidating effects of DM as well as associated vascular complications are classified into four categories viz.: nephropathy, retinopathy, neuropathy and cardiovascular disease. Hyperglycemia, which is associated with uncontrolled DM, elicits abnormal metabolism such that the enzymes involved in metabolic events leading to diabetic complications are expressed and amplified. The disorders associated with DM are linked to various metabolic pathways facilitated by enzyme activities of the polyol pathway, hexosamine biosynthetic pathway, glucose autoxidation as well as increased synthesis of advanced glycation end-products (AGEs), hexokinase-2 driven glycolytic overload, increased activities of the cyclooxygenase (COX), lipoxygenase (LOX) and pyruvate kinase (PKC) enzymes. The inhibition of the enzymes involved in these pathways could serve to mitigate and arrest diabetic complications.
Conclusion: Thus, suitable inhibitors for enzymes involved in DM metabolic events could serve as panaceas against DM complications, which will add to the growing list of new and more efficacious antidiabetic drugs.
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disorder that is characterized by hyperglycemia and glucose intolerance. It is known to be associated with impaired insulin secretion and peripheral sensitivity, as well as eventual β-cell dysfunction1. DM is one of the oldest diseases worldwide2. The International Diabetes Federation report of 2017 suggested that 451 million adults globally had diabetes in 2017, and 693 million individuals are expected to suffer from DM by 20453. The World Health Organization (WHO) also estimates that more than 19% of the world's total adult population will suffer from DM by the year 20304. DM has been a problem of great concern over the years due to its high incidence and mortality rates, as well as its high management and treatment costs3. DM disorders are more rampant in developing nations, with more than half of the total cases undiagnosed1.
DM is classified into type 1 and type 2. However, DM can also occur during pregnancy- a type known as gestational DM. Other circumstances- such as insulin receptor impairment, pancreatic exocrine disorder, genetic disorders, and endocrinopathies- can provoke DM2. Clinically, type 1 DM presents as hyperglycemia as a result of acute or chronic insulin deficiency in plasma5. In type 2 DM, the β-cells within the islets of Langerhans of the pancreas are hypersensitive to glucose in plasma, thereby eliciting the secretion of higher than normal insulin levels in the systemic circulation. The evidence of hyperinsulinemia is an attempt to counterbalance hyperglycemia, which further deteriorates and impairs β-cell function6, 7. Chronic hyperglycemia is accompanied by high mortality and morbidity due to its concomitant microvascular complications, such as nephropathy, neuropathy and retinopathy, as well as macrovascular complications which include cardiovascular diseases leading to myocardial infarction and stroke2, 8.
Hyperglycemia, which is associated with uncontrolled DM, elicits abnormal metabolism such that the enzymes involved in the metabolic events leading to diabetic complications are expressed and amplified8. Therefore, such enzymes can serve as therapeutic targets for the treatment of DM9. This review summarizes the major metabolic pathways leading to both microvascular and macrovascular complications in DM, and highlights the potential inhibition of the enzymes facilitating these processes as an instrument of diabetic control.
METHODS
Evidence acquisition
Data summarized in this review were sourced online from scientific search engines, including Google Scholar, Scopus, EMBASE, PubMed, ResearchGate, Mendeley, Medline and SpringerLink, using keywords such as 'diabetic complications', 'hyperglycemia-induced diabetic mechanisms', 'diabetic enzymes', and 'diabetic enzyme inhibitors'. A total number of 109 references published online between 1990 and 2020 were evaluated and cited in this review.
RESULTS
Diabetic complications
A significant number of complications accompany DM. However, the most dilapidating effects of DM and its associated vascular complications are classified into four categories, viz.: nephropathy, retinopathy, neuropathy, and cardiovascular disease2.
Nephropathy
Diabetic nephropathy is the main initiator of end-stage renal failure in the Western regions of the world10. Poor glycemic control is a risk factor for the occurrence of diabetic nephropathy11. Clinically, nephropathy is accompanied by an emergence of proteinuria with a concomitant reduction in glomerular filtration rate, leading to fatal uremia if not treated. Kidney disease is also characterized by macrovascular complications, including strokes and heart attacks2. According to Amico and Klein12, a rise in blood pressure is also associated with the onset of nephropathy.
Retinopathy
Diabetic retinopathy is the major cause of blindness in individuals between the ages of 20 – 74 years13, 14 since it initiates an array of lesions in the retina. It is typically characterized by vascular permeability changes, capillary degeneration, capillary microaneurysms, and abnormal production of blood vessels. Color vision deficiency is also another common effect of retinopathy13.
According to Forbes and Cooper2, hyperglycemia induces alteration in the blood-retinal barrier and its vascular permeability at the early stages of diabetic retinopathy. However, the visual disorders that occur at this stage are not noticeable to most sufferers.
Neuropathy
Diabetic neuropathy involves the destruction of the nerves and is one of the most prevalent diabetic complications. More than half of diabetic patients suffer from neuropathy15, 16. Diabetic neuropathy is the main risk factor for wound healing impairment commonly encountered in DM2. According to Obrosova 17, advanced diabetic neuropathy, as a result of impairment of the nerve fiber, leads to a total decline in sensory perception. Other complications associated with diabetic neuropathy include erectile dysfunction, cardiovascular dysfunction, paresthesia, hyperalgesia and allodynia2, 17.
Cardiovascular disease
There is a high prevalence of cardiovascular disease among individuals suffering from DM2. Cardiovascular disease is responsible for more than half of the total number of deaths recorded as a result of diabetic complications18. The risk of myocardial infarction among diabetic patients was equivalent to normal human subjects with a previous history of myocardial infarction19. The major disorders associated with cardiovascular disease among diabetic individuals include premature atherosclerosis, accompanied by myocardial infarction, stroke, and cardiac dysfunction2.
Furthermore, cardiovascular disease in type 1 DM occurs sequentially to obstruction in kidney function20, 21. In the same manner, poor glycemic control and kidney disease can provoke cardiovascular disease in type 2 DM22.
Metabolic pathways leading to complications in DM
Certain metabolic processes which are activated by hyperglycemia have been demonstrated to induce the complications associated with DM. These mechanisms, including enzymes and their intermediates, as well as inhibitors of these enzymes, are discussed below.
Protein kinase C (PKC) activation
The PKC family is comprised of more than eleven isoforms of serine-threonine kinases which play a major role in the modulation of endothelial cell permeability, activation of cell proliferation, and vascular growth23. PKC β is the core target in the escalation of diabetic disorders24, 25. The activation of PKC β in diabetic animals and vascular cells is initiated by hyperglycemia24, 25. An increase in blood glucose levels is accompanied by PKC activation in various tissues, including heart, retina, and renal glomeruli, which eventually exacerbates diabetic complications in both humans and animal models (Figure 1)26, 27, 28. High blood glucose levels directly activate the polyol pathway, whereas PKC associated with the polyol pathway is known to induce diabetic complications. Accordingly, the polyol pathway is linked with the generation of oxidative stress, leading to the emergence of diabetic complications as observed clinically (Figure 2)29, 30, 31. Additionally, continuous PKC activation stimulates different growth factors, including transforming growth factor β, platelet-derived growth factor, and vascular endothelial growth factor32, 33.
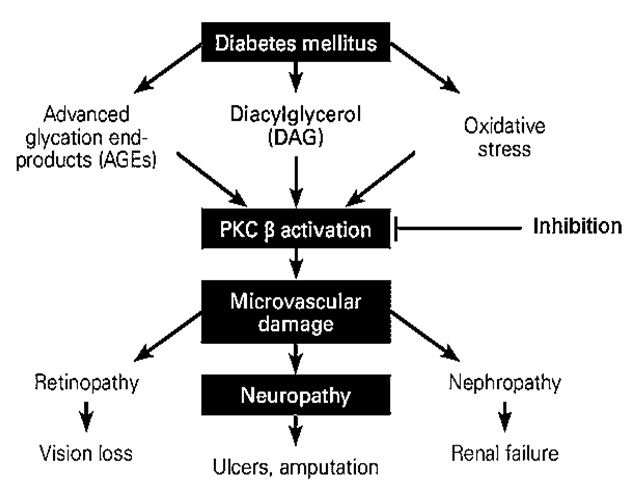
Diabetic complications arising from the activation of PKC β
The study carried out by Thomas .,34 on the protein kinase inhibitory activity of crude methanol extract of using hyphae formation inhibition assay against at 20 μg/disk concentration, yielded positive results. The alkaloids- namely chelerythrine and berberine- were reported in this plant extract and were responsible for the protein kinase inhibitory effects of this plant34.
The increased flow of the polyol pathway
The polyol pathway flux consists of two key enzymes: aldose reductase (AR) and sorbitol dehydrogenase (SD). In the polyol pathway, glucose is reduced to sorbitol (alcohol) by AR, followed by the oxidation of sorbitol to fructose by SD. Both the AR- and the SD-catalyzed steps involve using nicotinic acid adenine dinucleotide phosphate (NADPH) (Figure 2)35. The rate of the polyol pathway is dependent on the AR step; AR possesses low glucose affinity (Km > 100 mM) in nondiabetic individuals with normal glucose levels. Indeed, glucose metabolism via the polyol pathway involves the utilization of a very small amount of glucose36.
Under hyperglycemic conditions, AR is activated by rising intracellular glucose levels. The AR reaction eventually leads to the generation of springy polar sorbitol, which permeates the cell membranes, resulting in the distortions of cellular structure and activity, osmotic cell swelling, and reduction in ATPase function23. The oxidation of sorbitol activates PKC to fructose- a process catalyzed by SD- via a rise in NADH/NAD ratio37. Oxidative stress is generated in the polyol pathway via redox imbalance38, 39. Thus, the polyol pathway is associated with a vast array of diabetic complications (Figure 2).
Emodin, aurantio-obtusin and chryso-obtusin-2--β-D-glucoside, isolated from the ethyl acetate soluble extract of seeds elicited inhibitory effects on AR, with corresponding IC of 15.9, 13.6 and 8.8 μmol/L, respectively, against AR in rat lens40. Out of six phytocompounds isolated from roots, only isoliquiritigenin gave a strong inhibitory effect on AR as well as blocking the buildup of sorbitol in tissues of diabetic rats41. According to the inhibitory study carried out by Jung .,42 twelve phenolic compounds were isolated from rhizomes, among which tectorigenin and tectoridin gave the most potent and highest inhibitory effect (ICs = 1.12 and 1.08 μmol/L, respectively). Phenolic compounds blocked the accumulation of sorbitol in streptozotocin-induced diabetic rats within a period of 10 days at a dose of 100 mg/kg42. The AR inhibitory effect of luteolin isolated from at IC was 0.5 μmol/L43. The phytocompound, 3, 5-dicaffeoylquinic acid (chlorogenic acid derivative), from roots produced a substantial inhibitory effect on AR from rat lens44. Other phytocompounds with AR inhibitory effect include isoaffinetin from , rhetsinine from hot water extract of , matteuorienate A and B from rhizome47, puerariafuran from , and hypolaetin from -butanol extract49.

The polyol pathway of glucose metabolism (Modified from:
The increased flow of the hexosamine biosynthetic pathway
The contribution of the hexosamine biosynthetic pathway in the emergence of insulin resistance, as well as diabetic vascular complications, has been reported1, 51. This pathway involves the conversion of fructose-6-phosphate (fruc-6-P) to glucosamine-6-phosphate (glucN-6-P) using glutamine as the amino donor. The conversion of fruc-6-P to glucN-6-P is catalyzed by glutamine: fructose-6-phosphate-amidotransferase (GFAT), which is the rate-limiting enzyme of the hexosamine biosynthetic pathway. GlucN-6-P is instantly channeled towards the synthesis of uridine-5-diphosphate-N-acetylglucosamine (UDP--acetylglucosamine). The UDP-N-acetylglucosamine is the precursor for the biosynthesis of the necessary amino sugars required for the generation of glycoproteins, proteoglycans, glycosaminoglycans, and glycolipids51, 52, 53. Extremely high blood sugar levels induce the development of diabetic complications through the elevation of fruc-6-P concentration, which flows into the hexosamine biosynthetic pathway54, 55. However, increased blood glucose concentration induces metabolic pathways that eventually promote the release of cytokines such as TGF-β, ICAM-1, VCAM-1, TNF-α, CTGF and PAI-1, involved in various diabetic complications (Figure 3)56, 57. For instance, transforming growth factor-β1 (TGF-β1) plays a significant role in diabetic nephropathy51. Upon cellular glucose uptake, relatively larger glucose concentrations are catabolized and channeled towards glycogenesis, glycolysis, and pentose phosphate metabolism. Moreover, about 2-3% of glucose molecules are channeled into the hexosamine biosynthetic pathway57, 58.
Nevertheless, the inhibition of the rate-limiting enzyme, GFAT, of the hexosamine biosynthetic pathway blocks the hyperglycemic-induced transcription of the cytokines, thereby preventing the various diabetic complications which might possibly arise from the pathway51, 53, 54, 59.
The photo components present in fenugreek extracts possess an inhibitory effect against the rate-limiting enzyme pathway of the hexosamine biosynthetic pathway60. Diabetic mice fed with fenugreek-containing food showed an inhibited GFAT activity, whereas those given starch diets without fenugreek exhibited an increase in GFAT activity compared with the control group60. The anti-hyperglycemic potency of via the inhibition of GFAT has also been reported. The following phytocomponents from ,namely β-amyrin, taraxerol, 1--galloyl-β-D-glucose, corilagin, cosmosiin, quercetin-3-galactoside, and quercitrin, all exhibited inhibitory efficacy against GFAT with an absolute binding energy of > 8 kcal/mol61.

The hexosamine biosynthetic pathway showing the production of the cytokines including TGF-β, ICAM-1, VCAM-1, TNF-α, CTGF, PAI-1, involved in various diabetic complications (Modified from:
Increased synthesis of advanced glycation end-products (AGEs)
AGEs are yellowish-brown fluorescent substances. They are generated via the Maillard reaction. Specifically, they are produced via the non-enzymatic reaction between reducing sugars (e.g., glucose) and the amino group of proteins, leading to synthesis of a Schiff base. The resultant adducts are transiently converted to amadori compounds63. The amadori adducts undergo irreversible dehydration and condensation reactions to yield AGEs63, 64. AGEs are also synthesized from dicarbonyl compounds such as methylglyoxal, 3-deoxyglucosone, and glyoxal, which are outcomes of glucose autoxidation and degradation (Figure 5). Indeed, α-hydroxy aldehydes (including glycolaldehyde and glyceraldehyde) are also precursors for AGE synthesis65, 66. It has been shown that there is increased synthesis and accumulation of AGEs under chronic hyperglycemic conditions, leading to diabetic vascular complications63.

Advanced glycation end-products (AGEs) mechanism in diabetic vascular complications (Modified from:
The presence of AGEs induces the expression of AGE receptors. The interaction between AGEs and their receptors elevates cellular generation of oxidative stress, enhances the release of cytokines and growth factors via nuclear factor κB activation, and stimulates adhesion factors, all of which eventually lead to inflammatory response67. This interaction between AGEs and their receptor can furthermore escalate arteriosclerosis progression (Figure 4)64, 68. Aggravations of pathological angiogenesis, reduction in fibrinolytic activity, unstable angina, and/or acute myocardial infarction are other complications associated with an increase in AGE levels69, 70, 71.
Drugs such as atorvastatin, pravastatin, telmisartan, ramipril, rosiglitazone, exendin-4 and aminoguanidine have been reported to elicit modulatory effects on the diabetic complications caused by AGEs72, 73, 74, 75, 76, 77, 78. Atorvastatin, an antioxidant, is known to block the production of AGEs76. Pravastatin is involved in reducing tubular cell destruction in diabetic nephropathy and mitigates cell apoptosis initiated by the AGEs78. The expression of the AGE receptor was blocked by telmisartan in renal mesangial cells, endothelial cells, and liver cells74. Telmisartan also ameliorates the production of oxidative stress, inflammation, and arteriosclerosis associated with AGE expression74, 77. Previously, it has been reported that ramipril elicits similar effects as telmisartan75. In addition, rosiglitazone mitigates the expression of AGE receptor, while exendin-4 inhibits the development of diabetic nephropathy by blocking the interaction between AGEs and their receptors in tubular cells. The inhibition of AGE formation by aminoguanidine has also been reported72, 73.
Glucose autoxidation
Hyperglycemia exacerbates the glucose autoxidation process, which leads to the production of harmful reactive species and ketoaldehyde compounds. Specifically, peroxide (HO) and malondialdehyde are generated through this mechanism79.
Hyperglycemia increases the levels of reactive carbonyl species, such as methylglyoxal and glyoxal, as a result of glucose autoxidation54, 80. These reactive carbonyl species can preferentially undergo reactivity with arginine and lysine at relatively high rates (Figure 5), thereby provoking DM pathogenesis. Most of the protein binding sites consist of a large number of arginine residues81, 82. Furthermore, this metabolic process is also linked with the release of AGEs82.
Generally, the aldehyde group of glucose undergoes reactivity with the ε-amino groups of lysine residues and the N-terminal α-amino groups of proteins to produce a Schiff base. Subsequently, it undergoes rearrangement to yield an amadori intermediate. The amadori intermediate is further rearranged for the synthesis of heterogeneous AGEs. The arginine residues of proteins are structurally altered by reactive carbonyl species (Figure 5)82.

Synthesis of reactive carbonyl species that modify proteins and advanced glycation end products by glucose autoxidation

COX-2 activation by hyperglycemia (Modified from:
Increased expression of cyclooxygenase (COX)
Over the years, COX has been known to exist in the cells of mammals in only two isoforms, namely COX-1 and -283. However, a third isoform known as COX-3 was recently established, although its clinical significance has not been fully confirmed84. COX-1 is the most abundant isoform and occurs in almost all tissues83. COX-2 is released in trace amounts and is induced by growth factors, PKC activation, inflammatory cytokines, oxidative stress, and tumor promoters85, 86, 87.
An increase in COX-1 levels has been linked with DM onset, resulting in heart-related disorders with high mortality rates88. Furthermore, increased expression and activation of COX-2 have been linked with hyperglycemia through glucose autoxidation and AR pathway activation. This pathway is accompanied by secondary NADPH and NAD reduction, activation of PKC, stimulation of advanced glycated end-products receptor (RAGE), as well as elevation in reactive oxygen species generation (ROS) (Figure 6)83. According to a study carried out by Guo .,89 using type 2 diabetic mice, high COX-2 level was observed in the vascular smooth muscle cells of the mice. An elevation in the abundance of COX-2 in coronary arterioles was also observed and confirmed in diabetic human subjects90, 91. High COX-2 level in the podocytes was also observed and subjected the kidneys to diabetic glomerular injury through a (pro) renin-associated mechanism92.
Inhibition of COX-2 expression was reported to arrest nephropathy in diabetic subjects93, 94, 95. In a related study, the inhibitory action of nimesulide against COX-2 improved endothelial dysfunction in the hind limb vasculature of streptozotocin-induced diabetic rats96. The inhibitory effect of nonsteroidal anti-inflammatory drugs on COX activity has also been extensively reported97, 98.
Activation of lipoxygenase (LOX)
Lipoxygenases (LOX) is a family of enzymes typically characterized by non-heme iron-containing structures and is involved in the catalysis of polyunsaturated fatty acid deoxygenation arachidonic acid to generate hydroperoxyl derivatives, such as hydroperoxy-eicosatetraenoic acids (HPETEs)99.
The 12-LOX (an isoform of lipoxygenase) is activated by hyperglycemia and free fatty acids or pro-inflammatory cytokines. Furthermore, 12-LOX stimulation promotes the release of the pro-inflammatory lipid intermediates, 12 (S)-hydroperoxyeicosatetraenoic acid {12(S)-HPETE} and 12 (S)-Hydroxyeicosatetraenoic acid {12(S)-HETE}. These pro-inflammatory lipid intermediates- in concert with NADPH oxidase (NOX), p38 mitogen-activated protein kinases (p38-MAPK), and c-Jun N-terminal kinase (JNK)- can initiate the activation of inflammatory pathways. Eventually, these signaling pathways cause an elevation in ROS, oxidative stress, and endoplasmic reticulum (ER) stress, which can finally lead to impairment and death of β-cells100. The inhibition of the nuclear factor erythroid 2–related factor 2 (Nrf2) translocation by 12(S)-HETE, which controls the expression of antioxidants, is illustrated in Figure 7100, 101.
ML355 has been reported to be a potent inhibitor of human 12-LOX with an IC of 290 nm102. According to Adili 103 ML355 inhibited 12-LOX oxylipin production in a dose-dependent manner.

Role of 12-LOX in diabetic complications

Downstream diabetic consequences of hexokinase-2 driven glycolytic overload. (Modified from:
Hexokinase-2 driven glycolytic overload
Hexokinase-2 is the rate-limiting enzyme that catalyzes the first step of glycolysis, involving the phosphorylation of glucose to glucose-6-phosphate (G6P)105. Under hyperglycemic conditions, hexokinase-2 initiates an abnormal rise in glycolytic metabolic flux without concurrent transcriptional or other functional regulation. The metabolic flux leads to an unusual increase in the level of glycolytic intermediates. This process is known as a glycolytic overload and is accompanied by several diabetic complications104. The diabetic pathogenic mechanisms associated with this glycolic overload are as follows: G-6-P induces mitochondrial dysfunction, fruc-6-P is channeled to the hexosamine pathway, and dihydroxyacetone phosphate (DHAP) activates PKC. At the same time, AGEs are formed from methylglyoxal (MG) through glyceraldehyde-3-phosphate and DHAP intermediates (Figure 8)104, 106, 107, 108.
However, a possible therapeutic approach towards the prevention of the complications that may arise from glycolytic overload is through the inhibition of G6P buildup and hexokinase-2 displacement from the mitochondria. This can be made possible by channeling G6P towards the pentose phosphate pathway through the stimulation of glucose-6-phosphate dehydrogenase. This process also helps in mitigating carbohydrate response element (ChoRE)-linked expression of hexokinase-2109.
CONCLUSION
The disorders associated with DM are linked to various metabolic pathways, facilitated by enzyme activities of the polyol pathway, hexosamine biosynthetic pathway and glucose autoxidation, as well as being associated with increased synthesis of AGE hexokinase-2 driven glycolytic overload and increased activities of COX, LOX and PKC enzymes. The inhibition of the enzymes involved in these pathways could serve to mitigate and arrest diabetic complications. Thus, suitable inhibitors for enzymes involved in DM metabolic events could serve as panaceas against diabetic complications, and would add to the growing list of new and potentially more effective antidiabetic drugs.
Abbreviations
12-HETE: 12(S)-Hydroxyeicosatetraenoic acid
12-HPETE: 12(S)-hydroperoxyeicosatetraenoic acid
AGEs: Advanced glycation end-products
AR: Aldose reductase
Arg: Arginine
ChoRE: Carbohydrate response element
COX: Cyclooxygenase
DHAP: Dihydroxyacetone phosphate
DM: Diabetes mellitus
eNOS: Endothelial nitric oxide synthase
ER: Endoplasmic reticulum
F-1, 6-bis-P: Fructose-1, 6-bisphosphate
FFAR: Free fatty acid receptor
Fruc-6-P: Fructose-6-phosphate
G6P: Glucose-6-phosphate
GA3P: Glyceraldehyde-3-phosphate
GFAT: Glutamine: fructose-6-phosphate-amidotransferase
Gln: Glutamine
Glu: Glutamate
GLUT1/2: Glucose transporter 1 or 2
IL: Interleukin
JNK: c-Jun N-terminal kinase
LOX: Lipoxygenase
Lys: Lysine
MCP1: Monocyte chemoattractant protein 1
MG: Methylglyoxal
NADPH: Nicotinamide adenine dinucleotide phosphate
NF-kB: Nuclear factor-kappa B
NF-κB: Nuclear factor κB
NOX: Nicotinamide adenine dinucleotide phosphate oxidase
Nrf2: Nuclear factor erythroid 2–related factor 2
OGT: O-linked N-acetylglucosamine (O-GlcNAc) transferase
p38-MAPK: p38 mitogen-activated protein kinases
PGE2: Prostaglandin E2
PKC: Protein kinase C
PLA: Phospholipase A2
RAGE: Advanced glycation end-product receptor
ROS: Reactive oxygen species
SD: sorbitol dehydrogenase
TNF-α: Tumor necrosis factor α
TPI: Triosephosphate isomerase
UDP: Uridine diphosphate
UDP--acetylglucosamine: uridine-5-diphosphate-N-acetylglucosamine
Acknowledgments
None.
Authors' contributions
FOO/PCC conceived and designed the scope of the report. FOO/PCC/CMC contributed in writing the paper. FOO/PCC revised and edited the manuscript draft. FOO/PCC/CMC authors were the resource persons who provided all the necessary materials for writing the manuscript. FOO/PCC/CMC approved the manuscript in the present form and gave permission to submit the manuscript for publication.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

