
Utility of plasma galectin-3 in predicting long-term mortality in patients with acute heart failure
- Department of Internal Medicine, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
- Department of Cardiology, Cho Ray hospital, Ho Chi Minh City, 70000, Vietnam
Abstract
Background: Several studies have investigated Galectin-3 as a promising biomarker for predicting the short-term and long-term mortality of patients with acute heart failure. This study aimed to examine the usefulness of plasma galectin-3 at the time of admission in predicting long-term mortality in Vietnamese patients with acute heart failure (AHF).
Methods: We carried out a cohort study including 117 patients consecutively diagnosed with acute heart failure in a single cardiology department. Plasma galectin-3 and other biomarkers were measured at the time of admission. The patient’s clinical and analytical characteristics were recorded. The main endpoint was one-year all-cause mortality.
Results: There were six patients (5%) lost to follow-up and 59 patients (53.2%) reaching primary outcome within one year after hospital admission. The median plasma galectin-3 level (ng/mL) in patients with acute heart failure was 34.6 (26.7 – 44.1). Plasma galectin-3 in the alive group was significantly higher than that in the deceased group at one-year follow-up. In predicting one-year all-cause mortality, galectin-3 had an area under the curve (AUC) of 0.71 (95% confidence interval (CI), 0.62 – 0.81), representing a good prognostic factor, while brain natriuretic peptide (BNP) and troponin I were inferior to galectin-3 with an AUC of 0.69 (95% CI, 0.59 – 0.79) and 0.63 (95% CI, 0.53 – 0.74), respectively. The optimal cut-off value for galectin-3 was 40.75 ng/mL with a sensitivity of 50.1% and a specificity of 88.5%. In a multivariate model, patients with galectin-3 levels > 40.75 ng/mL had a hazard ratio (HR) of 2.8 (95% CI, 1.5 – 5; p = 0.001). The best prediction model was the combined model of galectin-3 and BNP, yielding an AUC of 0.78 (95% CI, 0.70 – 0.86; p < 0.001).
Conclusions: Our study suggested that galectin-3 levels could predict long-term all-cause mortality in patients with acute heart failure with a good prognostic capacity. Combining galectin-3 and BNP could yield better risk stratification.
Introduction
It is estimated that 26 million people worldwide live with heart failure, which represents a global health problem. It is also expected that this number will rapidly increase due to the population aging in general and the growing prevalence of comorbidities1. Over the past decade, the utilization of different biomarkers in evaluating heart failure has gained an essential clinical value, and serves as an essential tool for diagnosis, prognosis, and even therapy-guiding or decision making2. Accordingly, the use of natriuretic peptides including brain natriuretic peptide (BNP) or N-terminal pro-brain natriuretic peptide (NT-proBNP) were recommended at class IA in the current American and European guidelines for heart failure, for either diagnostic or prognostic purposes3, 4. Although they have an excellent ability to differentiate and identify acute heart failure patients, the prognostic capacity of natriuretic peptides remains average. Moreover, natriuretic peptides values are affected greatly by several factors, such as age, renal function, and obesity status5, 6, 7. Therefore, novel biomarkers are being studied with the aim of adding benefits with respect to prognostication and risk stratification.
Galectin-3 is a soluble beta-galactoside binding protein produced predominantly by activated fibroblasts and macrophages and serves as a pro-fibrotic and pro-inflammatory biomarker8. Galectin-3 initiates the fibrotic process by activating macrophages and fibroblasts, resulting in the release of several pro-inflammatory molecules, leading to tissue injury, inflammation, enhanced collagen deposition, and extra-cellular matrix adhesion9. The role of galectin-3 in heart failure was first reported by Sharma et al. The injection with galectin-3 into the pericardial sac of normal rats resulted in cardiac remodeling with cardiac dysfunction over-expression of collagen10. Recently, several studies have shown that galectin-3 is not just an innocent onlooker but a pivotal player in the pathophysiology of heart failure, in which inhibition of galectin-3 in several models of heart failure could reverse heart fibrosis11, 12, 13, 14. As a biomarker, galectin-3 showed potential value in the prognostication of HF patients15. Indeed, van Kimmenade also showed that galectin-3 surpassed NT-proBNP in predicting 60-day mortality and rehospitalization16. Recently, the American Heart Association guidelines have included the recommendation at class IIb to assess galectin-3 level either for prognosis or risk stratification in chronic and acute heart failure patients4, 17. However, data regarding the capacity of galectin-3 to predict all-cause mortality in AHF patients, especially in the Asian population, are scarce. We only found a single study including Chinese hospitalized patients with acute heart failure which showed that the utility of galectin-3 alone as a prognostic factor was not statistically significant for predicting the sudden or in-hospital death18. A few other reports investigated 30-day, 60-day and all-cause mortality following an episode of acute heart failure, but they used serial galectin-3 measurements16, 19, 20. We designed this study to investigate whether plasma galectin-3 measured at the time of admission could allow one-year mortality prediction. Our study was carried out to provide further evidence and a better understanding of the predictive ability of galectin-3 in the Vietnamese population.
Methods
Study design
This cohort study was conducted in the Department of Cardiology, Cho Ray Hospital, Ho Chi Minh City, Vietnam. We recruited 117 consecutive patients diagnosed with acute heart failure from March 2018 to July 2018, based on the European Society of Cardiology (ESC) criteria3. Interventions, treatments, and discharge were carried out according to the hospital protocols. The study was conducted in compliance with the Declaration of Helsinki. The Ethical Committees of Cho Ray Hospital approved it on February 12, 2018 with the number of approval letters being 63/DHYD-HD. All subjects or relatives signed a written informed consent to participate in the present study.
For each subject in our study, epidemiological characteristics were recorded, which included demographic variables (age and sex), comorbidities and risk factors (hypertension, smoking, diabetes, atrial fibrillation, ischemic heart disease, and previous episodes of acute heart failure), body mass index, New York Heart Association function status, signs (systolic pressure, diastolic pressure, heart rate, peripheral edema, pulmonary rales), estimated glomerular filtration rate, biomarkers (galectin-3, BNP, troponin I, and albumin), and echocardiographic parameters (left ventricular ejection fraction; LVEF). The estimated glomerular filtration rate (eGFR) was calculated according to the 2009 CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) creatinine equation: eGFR (mL/min/1.73 m) = 141 × min(serum creatinine[mg/dL]/ κ, 1) × max(serum creatinine[mg/dL]/ κ , 1) × 0.993 × 1.108 [if female] × 1.159 [if black], with κ = 0.7 (female) or 0.9 (male), α = -0.329 (female) or -0.411 (male), and age in years21. All biochemical parameters and biomarkers measurements were performed at the time of admission. The quantitative two-dimensional (biplane Simpson) method was used to measure left ventricular ejection fraction (LVEF) during the hospital stay. We followed up patients for one year from hospitalization to investigate and assess one-year all-cause mortality, which was the endpoint of the study.
Galectin-3, BNP, Troponin I, and Albumin assay
At the time of admission, blood samples were collected into tubes containing ethylenediaminetetraacetic acid and then transported to the Biochemistry Department of Cho Ray Hospital to be processed and frozen at -80ºC for eventual measurements of galectin-3, BNP, troponin I, and albumin. Galectin-3 was analyzed using ARCHITECT galectin-3 assay, which is a two-step immunoassay procedure (ARCHITECT galectin-3, Abbott, Park, IL, USA) on the Architect i2000SR analyzer (Abbott Laboratories, Park, IL, USA), according to the manufacturer's instructions22. BNP was analyzed using ARCHITECT BNP (ARCHITECT BNP, Abbott) assay on the Architect i2000SR analyzer (Abbott Laboratories). Troponin I was analyzed using chemiluminescent microparticle immunoassay on the ADVIA Centaur XP analyzer (Siemens Diagnostics, Tarrytown, NY, USA). Albumin was analyzed using the colorimetric method on the ADVIA 1800 analyzer (Siemens Diagnostics, Tarrytown, NY, USA).
Statistical analysis
Continuous variables were presented as mean and standard deviation (SD) for the quantitative variables or as median and interquartile range (IQR) for those without a normal distribution, and as absolute values and percentages for the qualitative variables. Pairwise comparisons between patients with acute heart failure achieving or not achieving the endpoint were performed using the Student’s T-test or non-parametric Mann-Whitney U test for continuous variables, or chi-square test or Fisher’s exact test for categorical variables, where appropriate. Univariable logistic regression was used to evaluate how well galectin-3 and BNP predicted the outcomes. We then used the multivariable logistic regression for the covariates of galectin-3, BNP, and other factors in predicting the outcomes. The variable selection was performed with Bayesian model averaging (BMA) method23. Receiver-operating characteristic (ROC) curves were also derived to evaluate the performance of galectin-3, BNP, and troponin I for predicting the endpoint in patients with acute heart failure. Differences were considered as statistically significant with a p-value less than 0.05, or when the 95% CI of the HR excluded the value of 1. All analyses were conducted using the Rstudio version 1.2 (PBC, Boston, MA).
Results
Patient characteristics
A total of 117 patients diagnosed with acute heart failure were included in the study. Six patients (5%) were lost to follow-up, and fifty-nine patients (53.2%) died within one year after hospital admission. The clinical and analytical characteristics of patients with acute heart failure are presented in
Clinical characteristics of patients with acute heart failure
|
Characteristics |
Summary statistic (N = 117) |
|
Age (year), mean ± SD |
66.2 ±17.5 |
|
Gender male, n (%) |
65 (55.6%) |
|
Smoking, n (%) |
57 (48.7%) |
|
Hypertension, n (%) |
73 (62.4%) |
|
Diabetes, n (%) |
36 (30.8%) |
|
Atrial fibrillation, n (%) |
11 (9.4%) |
|
Coronary artery disease, n (%) |
85 (72.6%) |
|
Chronic heart failure, n (%) |
86 (73.5%) |
|
Prior acute heart failure, n (%) |
63 (53.8%) |
|
BMI (kg/m2), median (IQR) |
22.0 (19.7, 23.9) |
|
Systolic blood pressure (mmHg), median (IQR) |
110 (100, 120) |
|
Diastolic blood pressure (mmHg), median (IQR) |
70 (60, 75) |
|
Heart rate (beats per minute), median (IQR) |
90 (80, 100) |
|
NYHA classification, n (%) | |
|
- Grade I |
0 (0.0%) |
|
- Grade II |
45 (38.5%) |
|
- Grade III |
59 (50.4%) |
|
- Grade IV |
13 (11.1%) |
|
Leg edema |
64 (54.7%) |
|
Pulmonary rales |
43 (36.8%) |
|
eGFR (ml/min/1.73 m2), median (IQR) |
49.6 (33.9, 61.9) |
|
Ejection fraction (%), median (IQR) |
27 (20, 33.5) |
|
Plasma albumin level (g/dl) median (IQR) |
3.3 (3.0, 3.6) |
|
Plasma troponin I level (ng/ml) median (IQR) |
0.79 (0.09, 7.85) |
|
Plasma BNP level (pg/ml), mean ± SD |
2675.3 ± 1263.2 |
|
Plasma galectin-3 level (ng/ml), median (IQR) |
34.6 (26.7, 44.1) |
|
1-year lost to follow-up |
6 (5.0%) |
|
1-year mortality, n (%) |
59 (53.2%) |
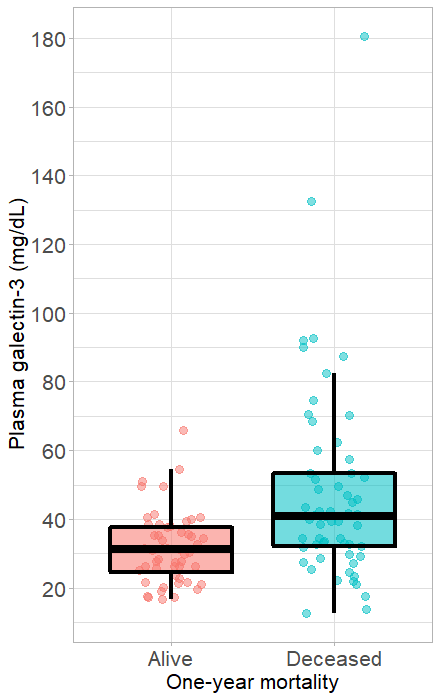
Plasma galectin-3 concentrations in the alive and deceased patients. Box plots showing the plasma galectin-3 levels in the alive and deceased patients (A) during hospitalization and (B) within one year of hospital admission. The upper and lower edges of each box represent the interquartile range (25th — 75th percentile) while the middle line is corresponding to the median. The points are the actual values of plasma galectin-3 level and colored by alive (in red) and deceased (in blue) patients.
Correlation of Galectin-3 and other biomarkers with one-year mortality
A total 59 AHF patients died within one year after hospital admission. In predicting one-year mortality, it was found that galectin-3 level in the deceased group was significantly higher than that in the alive group (Figure 1). Differences in demographic, medical history, and biomarker characteristics between the alive and deceased groups are presented in
Characteristics of the alive and deceased patients within one year from hospital admission
|
Characteristics |
One-year all-cause mortality | ||
|
No (N=52) |
Yes (N=59) |
p value | |
|
Age (year), mean ± SD |
63.1 ± 18.4 |
70.7 ± 14.9 |
0.018 |
|
Gender male, n (%) |
30 (57.7%) |
30 (50.8%) |
0.470 |
|
Smoking, n (%) |
25 (48.1%) |
29 (49.2%) |
0.910 |
|
Hypertension, n (%) |
31 (59.6%) |
39 (66.1%) |
0.480 |
|
Diabetes, n (%) |
12 (23.1%) |
21 (35.6%) |
0.150 |
|
Atrial fibrillation, n (%) |
8 (15.4%) |
3 (5.1%) |
0.070 |
|
Coronary artery disease, n (%) |
39 (75%) |
42 (71.2%) |
0.652 |
|
Chronic heart failure, n (%) |
37 (71.2%) |
44 (74.6%) |
0.685 |
|
Prior acute heart failure, n (%) |
27 (51.9%) |
33 (55.9%) |
0.672 |
|
Systolic blood pressure, median (IQR) |
120 (100, 120) |
110 (100, 120) |
0.513 |
|
Diastolic blood pressure, median (IQR) |
70 (60, 70) |
70 (60, 80) |
0.884 |
|
Heart rate (beats per minute), median (IQR) |
90 (80, 100) |
90 (80, 109) |
0.319 |
|
eGFR (ml/min/1.73 m2), median (IQR) |
56.9 (36.5, 63.9) |
41.8 (30.3, 55.7) |
0.001 |
|
Ejection fraction (%), median (IQR) |
27.5 (20, 34) |
26 (20, 33) |
0.703 |
|
Plasma albumin level (g/dL), median (IQR) |
3.4 (3.0, 3.8) |
3.2 (3.0, 3.5) |
0.010 |
|
Plasma troponin I level (ng/mL), median (IQR) |
0.2 (0.1, 2.4) |
1.2 (0.2, 10.4) |
0.016 |
|
Plasma BNP level (pg/mL), mean ± SD |
2267.6 ± 1131.3 |
3069 ± 1264.3 |
0.001 |
|
Plasma Galectin-3 level (ng/mL), median (IQR) |
31.2 (24.1, 38) |
41.1 (31.9, 53.5) |
< 0.001 |
Cox regression model for one-year mortality with linear effect of Galectin-3
|
Univariable analysis |
Multivariable analysis | |||||
|
HR |
95% CI |
P value |
HR |
95% CI |
P value | |
|
Galectin-3 levels (ng/ml) | ||||||
|
- Galectin-3 ≤ 40.75 |
1 |
- |
Ref |
1 |
- |
Ref |
|
- Galectin-3 > 40.75 |
3.47 |
2.07 – 5.83 |
<0.001 |
2.60 |
1.47 – 4.60 |
0.001 |
|
Age (year) |
1.02 |
1 – 1.04 |
0.027 |
1.006 |
0.99 – 1.03 |
0.524 |
|
eGFR (ml/min/1.73m2) |
0.98 |
0.96 – 0.99 |
0.006 |
0.99 |
0.98 – 1.01 |
0.318 |
|
Albumin (g/dL) |
0.45 |
0.27 – 0.76 |
0.003 |
0.51 |
0.27 – 0.96 |
0.037 |
|
Troponin I (ng/mL) |
1 |
1 – 1.005 |
0.090 |
1 |
0.99 – 1.005 |
0.108 |
|
BNP (pg/mL) |
1 |
1 – 1.001 |
0.002 |
1 |
1 – 1 |
0.185 |
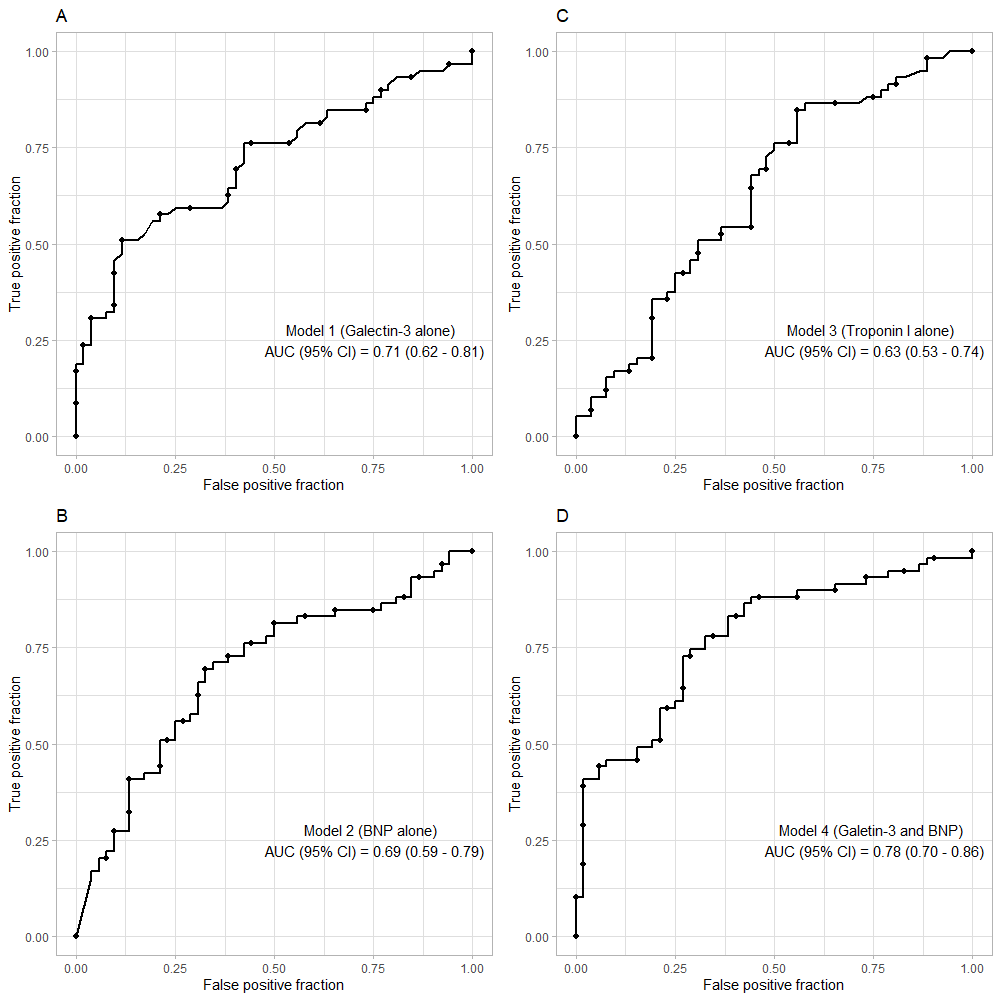
Receiver operating characteristic (ROC) curves for prediction of one-year all-cause mortality. Area under the curve of ROC (with the 95% confidence interval) for the prediction of one-year mortality for (A) galectin-3, (B) BNP, (C) Troponin I, and (D) Galectin-3 + BNP.
Our ROC curve analysis showed that plasma galectin-3 alone was a good prognostic factor for one-year mortality with the AUC (95% CI) of 0.71 (0.62 – 0.81) (Figure 2A). BNP and troponin I alone were not strong prognostic factors, with AUCs (95% CI) of 0.69 (0.59 – 0.79) and 0.63 (0.53 – 0.74), respectively (Figure 2B, C). The optimal cut-off value for galectin-3 for predicting one-year all-cause mortality was 40.75 ng/mL, with a sensitivity of 50.1% and a specificity of 88.5%. Furthermore, the AUC (95% CI) for the combined biomarkers (galectin-3 + BNP) was 0.78 (0.70 – 0.86), which was higher than the AUC of galectin-3 alone (Figure 2D). Based on the Kaplan-Meier analyses, patients with elevated galectin-3 and BNP had the highest rates of death, while patients with no elevated markers had the lowest rates (Figure 3).

Kaplan-Meier curve showed that acute heart failure patients with a plasma galectin-3 level more than 40.75 ng/mL and brain-natriuretic peptide level above the optimal cut-point 2549 pg/mL were associated with higher rates of mortality than either of the two markers alone.
Discussion
Our study showed that galectin-3 could predict one-year all-cause mortality with an area under the ROC curve of 0.71 (95% CI, 0.62 – 0.81), whereas BNP and troponin I were inferior to galectin-3 with AUCs (95% CI) of 0.69 (0.59 – 0.79) and 0.63 (0.53 – 0.74), respectively. Moreover, the combination of galectin-3 and BNP resulted in an improved prognostic value for one-year mortality with AUC of 0.78 (95% CI, 0.70 – 0.86).
Previous studies have investigated the contribution of galectin-3 in the pathogenic process of heart failure. Nguyen et al. suggested that the major factors contributing to the cardiac release of galectin-3 and elevated circulating levels of galectin-3 are cardiac inflammation and enhanced activation of β-adrenergic receptors24. Several studies have assessed the diagnostic capacity of galectin-3 and compared it to natriuretic peptides (BNP and NT-proBNP) in identifying acute heart failure patients. For example, van Kimmenade revealed that AUC of NT-proBNP was 0.94 for HF diagnosis in dyspneic patients, whereas the AUC of galectin-3 was 0.7416. Recently, studies have focused on discovering biomarkers of diagnostic capability in HF to biomarkers of prognostic value.
Our findings were supported by several studies that reported the usefulness of galectin-3 as a risk predictor of mortality in AHF patients. A large cohort study that included 1140 hospitalized heart failure patients showed that galectin-3 had a good prognostic value for in-hospital mortality with AUC of 0.71 (95% CI, 0.65 – 0.77)18. Besides, combining galectin-3 and another biomarker (NT-proBNP) resulted in an improved AUC of 0.81 (95% CI, 0.76 – 0.86). Another study by van Kimmenade .16showed that galectin-3 represents a remarkable biomarker for predicting 60-day all-cause death in 209 AHF patients admitted to the emergency setting. This study also displayed that increased levels of galectin-3 had an OR of 14.3 (95% CI, 5.6 – 45.1; p < 0.001) after adjustment for age, glomerular filtration rate, and New York Heart Association (NYHA) functional classification. Therefore, the authors concluded that galectin-3 was an independent prognostic factor for 60-day mortality and rehospitalization.
Similarly, Miró . 25showed that galectin-3 has a superior prognostic value (AUC = 0.732, p = 0.041) for predicting the 30-day mortality in 115 AHF patients compared to NT-proBNP (AUC = 0.586, p = 0.446) and troponin I (AUC = 0.639, p = 0.222). Miró . also showed that the concentrations of galectin-3 could be applicable for risk stratification of short-term mortality in the emergency setting. For example, patients with galectin-3 levels between 30–42 μg/L had an OR of 5.25 (95% CI, 0.53 – 51.63), whereas those with galectin-3 levels > 42 μg/L had an OR of 8.88 (95% CI, 1.49 – 52.73).
Intriguingly, Demissei. 26showed that the AUCs of galectin-3 measured at admission, day 3, day 7, and day 14 did not have any statistical difference in predicting the 180-day mortality in 2023 AHF patients. The serial measurement of galectin-3 during a hospital stay is not recommended. Accordingly, galectin-3 was measured only once for each patient in the study and at the time of admission in our study. To our knowledge, this is the first study assessing the prognostic ability of galectin-3 in predicting the long-term mortality in AHF patients in the Vietnamese population and the second in the Asian population. However, one of the limitations is the small sample size of AHF patients included in our study, which could have introduced a type II error. Besides, there was no matching control group to compare the circulatory levels of galectin-3 with the AHF group. Our study also did not assess the diagnostic capacity of galectin-3 in patients with AHF. Therefore, further studies with a more significant number of patients enrolled are warranted to evaluate the diagnostic and long-term prognostic utility of combining galactin-3 with other biomarkers, , soluble ST2 and high-sensitivity (hs) troponin.
Conclusion
Galectin-3 measured at the time of admission showed an excellent prognostic capacity to predict one-year all-cause mortality in patients with acute heart failure. The results of our study support the utility of a combined galectin-3 and BNP model as a risk predictor of long-term mortality in AHF patients.
Abbreviations
AHF: acute heart failure
AUC: area under the curve
BMA: Bayesian model averaging
BNP:brain natriuretic peptide
CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration
eGFR: Estimated glomerular filtration rate
HF: heart failure
IQR: inter quartile range
LVEF: left ventricular ejection fraction
NT-proBNP: N-terminal pro-brain natriuretic peptide
NYHA: New York Heart Association
ROC: Receiver-operating characteristic
SD: standard deviation
Acknowledgments
We thank the Ethical Committees of University of Medicine and Pharmacy at Ho Chi Minh city and Cho Ray hospital for approving this study; we thank Dr. Tran Thanh Vinh, head of Biochemistry Department of Cho Ray hospital, for performing galectin-3 and other biomarker assays; we thank Ms. Huynh Thi Lien Nga and Mr Dang Quang Loi for supporting this study.
Author’s contributions
Conceptualization and Methodology: H.V.S., D.Q.T, C.N.H; Data collection: H.V.S., D.Q.T., T.T.T.H.; Analysis: H.V.S., D.Q.T.; Writing original draft preparation: H.V.S., D.Q.T., T.T.T.H., C.N.H., T.T.T.; Writing, review, editing: H.V.S, D.Q.T. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review board of Cho Ray hospital approved the study with the number of approval letter being 63/DHYD-HD, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

