
In vivo study of wound healing processes in Sprague-Dawley model using human mesenchymal stem cells and platelet-rich plasma
- Klinik Hayandra, Yayasan Hayandra Peduli, Jakarta, Indonesia
- HayandraLab, Yayasan Hayandra Peduli, Jakarta, Indonesia
- Fakultas Kedokteran, Universitas Pembangunan Nasional Veteran Jakarta
- Pusat Kajian Stem Cell, Universitas Pembangunan Nasional Veteran Jakarta
- Department of Biology, Faculty of Mathematic and Natural Sciences, Mulawarman University, Samarinda, Indonesia
Abstract
Background: Mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP) have a potential role in improving wound healing processes. This experimental study aims to compare PRP and MSCs to promote the wound healing process in the animal burn wound model.
Methods: PRP from venous blood and MSCs from lipoaspirates were isolated from six donors. Saline solution was used as control while PRP and MSCs as treatment groups were injected to second-degree burn wounds into the backs of 10 male Sprague-Dawley rats for each group. On day-7, 5 rats from each group were euthanized for analyzing VEGF gene, which has roles in angiogenesis. At the end of the study (week 5), the remaining rats were euthanized for histological analysis.
Results: The VEGF expression in MSCs and PRP groups was higher than the control group (not significant). The wound healing rate was also faster until 21 days post-burn in the MSCs and PRP groups and getting slowly afterward. Histological analysis showed the burned skin at day 35 had displayed the best differentiation outcome in the MSCs group. In conclusion, human-derived MSCs and PRP do not accelerate epithelialization duration of rats burn wound model. However, they improved wound’s vascularization and cell differentiation.
Conclusion: MSCs are superior to PRP in enhancing cell differentiation.
Introduction
Mesenchymal stem cell (MSC) therapy has been utilized in many pathological conditions to stimulate tissue regeneration. Depending on the specific medium, MSCs can differentiate into several types of cells1. Previous studies reported the benefits of MSCs in the cases of calvarial defects, Crohn’s disease, critical limb ischemia, and multiple sclerosis, as well as in enhancement of wound healing2. Numerous studies have reported on the efficacy of autologous and allogeneic MSCs in applications to improve the wound healing process in various wound models2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15. The effectiveness of MSCs to promote wound healing has also been proven in xenogenic applications16, 17, 18, 19, 20.
Another well-known modality to improve the wound healing process is platelet-rich plasma (PRP) which contains various growth factors that facilitate the wound healing process21, 22, 23, 24, 25. PRP has shown excellent results in many studies as well as studies related to wound healing21, 26, 27, 28, 29, 30, 31, 32. PRP has also been studied for several clinical conditions, including diabetic foot ulcers, sport soft tissue injuries, and burn wounds, all with encouraging results33, 34, 35. The combination of PRP and MSCs can result in further improvement of MSC-mediated wound healing properties36, 37.
Even though MSCs have shown immense therapeutic benefit and potential, its clinical application is sometimes hindered by regulation issues and high processing costs38. Furthermore, processing and cell amplification have raised safety concerns about the risk of contamination, mutation, and differentiation capacity39, 40. Therefore, PRP could be an alternative to accelerate wound healing with relatively lower cost and simpler preparation techniques than MSCs. Blood collection in PRP-based therapy would also be more tolerable for critically ill patients than fat-harvesting procedures in autologous MSC preparations. Therefore, sometimes PRP becomes a more feasible option for patients.
Currently, there is limited evidence comparing the therapeutic efficacy of PRP and MSCs as a solitary treatment to improve wound healing. This experimental study aims to compare PRP and MSCs to promote the wound healing process in the animal burn wound model.
Methods
This experimental study was conducted in the Animal Hospital of Bogor Agricultural Institute Facility, in compliance with the protocol, and includes the use of human blood and human tissue that had been reviewed and approved by the Health Research Ethics Committee of the University of Indonesia and Cipto Mangunkusumo Hospital (HREC-FMUI/CMH), with letter no. 625/UN2.F1/ETIK/2016. This study complied with the ARRIVE guidelines and the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). Contact burn wounds were made on the back of 30 male Sprague-Dawley rats weighing between 100−175 grams. The rats were randomized into three groups: (1) human PRP treatment group, (2) human MSC treatment group, and (3) saline solution treatment group (control). MSCs and PRP were isolated from patients in our medical center. Informed consent was obtained from each patient. The patients underwent stromal vascular fraction (SVF) therapy to treat the various medical conditions described in
Demographic and medical data of PRP and MSCs donors
|
Donor |
Gender |
Age |
Therapeutic indication |
|
1 |
F |
75 |
Low back pain due to herniated nucleus pulposus |
|
2 |
F |
45 |
Anti aging |
|
3 |
M |
34 |
Autism |
|
4 |
F |
56 |
Metabolic syndrome |
|
5 |
F |
68 |
Anti aging |
|
6 |
M |
62 |
Anti aging |
PRP Preparation
Blood collection was done using sodium citrate tubes. Four tubes containing 4 mL of blood were centrifuged at 300 g for 5 minutes. Plasma was transferred into a new 15 mL conical tube, and centrifuged at 1000 g for 5 minutes. The upper layer of plasma was discarded until 5 cc remained at the bottom of the tube, along with the aggregate of the platelet. Platelet and plasma were mixed and activated with calcium activator (H-Remedy). Fibrin-free PRP was treated with the light activation method, as described by the Adistem method. Activated PRP was prepared 1–3 days before application to the wound and stored at 2 — 8⁰C.
MSC Preparation
Fresh lipoaspirate was obtained from the abdominal fat of human donors. Tissue-dissociation enzyme (H-Remedy) was added to the lipoaspirate in 50 mL tubes41. The mixture was incubated for 1 hour in a 37 C incubator. After incubation, low glucose Dulbecco's Modified Eagle's Medium (DMEM; Gibco, Waltham, MA, USA) containing 4 mM L-glutamine (Gibco) was added to inactivate the enzyme. Tubes containing digested lipoaspirate were centrifuged for 5 minutes at 600 g. Supernatant from each tube was discarded. SVFs in the form of cell pellets were collected, mixed with lysed red cell buffer, and incubated for 10 minutes at room temperature. Afterwards, SVFs were washed twice with phosphate buffered saline (PBS) pH 7.4, and diluted in saline solution. Some of the SVFs were separated for this study and cultured in 25 cm flasks with cell density set at 125,000 cells/flask and temperature set at 37 ˚C. The combination of low-glucose DMEM containing 4 mM L-glutamine supplemented with 10% FBS (Gibco), 1% antibiotic-antimycotic solution (10,000 units/mL of penicillin, 10,000 µg/mL of streptomycin, and 25 µg/mL of Amphotericin B) (all from Gibco), and 0.05 ng/mL L-ascorbic acid was used as the culture medium. The culture medium was replaced every 2−3 days with fresh medium. Cells were sub-cultured after they reached 80% confluency. Passage 1 MSCs were harvested and stored in saline solution at 4 ˚C for 1 − 3 days prior to use.
Flow cytometry
Cell surface marker analysis was performed by flow cytometry (Miltenyi Biotec, Auburn, CA, USA) to confirm the stem cell characteristics of ADSCs. The cell surface markers used were CD73 allophycocyanin (APC), CD90 fluorescein isothiocyanate (FITC), and CD105 peridinin-chlorophyll-protein (PerCP) Cy5.5, as positive MSCs markers. Lineage negative markers and/or positive hematopoietic cells markers included PE-conjugated CD34, CD45, CD11b and CD19, and human leukocyte antigen (HLA)-DR (Becton Dickinson, Franklin Lakes, NJ, USA). The cells (1 × 10, passage 3) were stained with fluorescence-labeled probes specific to the cell surface molecule. Data were obtained from 10,000 events per analysis.
Deep Partial Thickness Wound Healing Model and Therapeutic Modality Application
The wound healing model by Zhang . was used as a reference with minor modifications42. The animals were allowed 1 week of an adaptation period. Ketamine (100 mg/mL) and xylazine (0.4 mg/mL) were injected into the intraperitoneal space. The back of the rat was shaved and cleaned with povidone-iodine (10%). A hot flat-round bottom stainless steel (200 g) was placed at the shaved area without pressure to create a deep partial-thickness contact burn wound for 4 seconds. The hot metal had been previously immersed in 100 C water for 5 minutes. The heating process was repeated before each application. The rats were randomly assigned to three groups of wound treatment. Each group consisted of 10 rats. Group A received 500 L intradermal PRP injection at the burn wound. Group B received intradermal MSC injection with a total cell count of 4 x 10cells per rat. Group C received an intradermal normal saline injection and served as the control group. All of the rats received free access to food and water during the 5-week observation period.
Wound Healing Analysis
Macroscopic evaluation
All of the wounds were weekly inspected and photographed until the 5 week. Two blinded assessors evaluated the wound surface area by using image analysis software (Image J; National Institute of Health, Bethesda, MD, USA). Percentages of wound areas were calculated by dividing the remaining wound area with the initial wound area.
Real-time analysis of vascular endothelial growth factor (VEGF) expression
At day 7, rats (n = 5 from each group) were euthanized. All rats were killed using the cervical dislocation technique. The entire wound and surrounding intact skin were collected and processed to extract the DNA. Rat VEGF was detected and measured by real-time polymerase chain reaction (RT-PCR) method. The primer sequences of VEGF from rats were 5’-GTGTGGTCTTTCGTCCTTCTTA-3’ (forward) and 5’-GTTTGTCGTGTTTCTGGAAGTG- 3’ (reverse), while β-actin primer sequences were used as internal controls and were 5’- GTGTGGATTGGTGGCTCTATC-3’ (forward) and 5’-CAGTCCGCCTAGAAGCATTT-3’ (reverse). Total ribonucleic acid was extracted using QIAamp RNA Blood Mini Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s protocol. Complementary DNA (cDNA) was synthesized from RNA by QuantiTect Reverse Transcription Kit (Qiagen). The resultant cDNA was subjected to quantitative real-time PCR (qPCR) performed using QuantiTect SYBR Green PCR Kit (Qiagen).
Microscopic evaluation
At the end of the study (week 5), the remaining 14 rats were euthanized using the cervical dislocation technique. One rat from the MSC treatment group died during the trial but the death was considered to be unrelated to the MSC treatment. The entire wound and surrounding intact skin were collected and subjected to histological analysis by hematoxylin-eosin (H&E) staining. Tissue regeneration was analyzed based on re-epithelization and the presence of skin appendages (e.g. hair follicle and sebaceous gland).
Statistical Analysis
Data analysis was performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). One-way ANOVA test and Kruskal-Wallis test were used to analyze the difference of the wound area percentage between groups. The least significant difference (LSD) post-hoc analysis was performed when there was a statistically significant difference in the ANOVA test. Wound area closure progression over a period of time was also analyzed by repeated ANOVA. Alpha value was set at 0.05.
Results
Cell surface markers
The cells were confirmed as mesenchymal stem cells due to their expression of differentiation markers CD73, CD90, and CD105 (positive markers). CD45, CD34, CD11b, CD19 and HLA-DR were considered to be negative markers (Figure 1). The expression of CD73, CD90, and CD105 were 99.95%, 99.79%, and 98.61%, respectively, while lineanage negative expression (lin negative) was 1.90%.
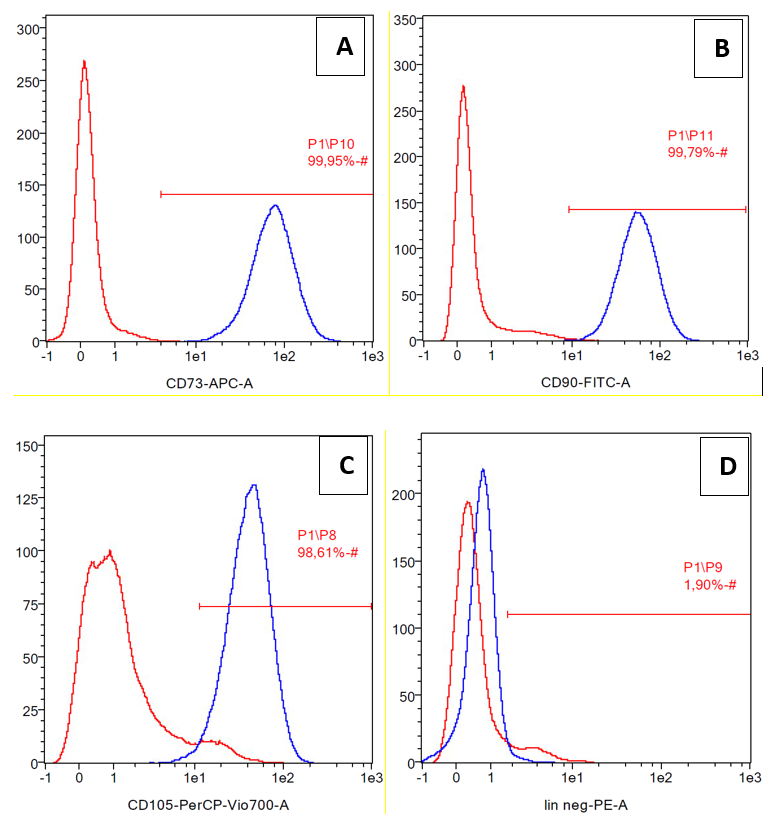
Cell surface marker expression of ADSCs, positive markers were CD73 (A), CD90 (B),CD105 (C) and lineage negative CD45, CD34, CD11b, CD19, HLA-DR (D).
Wound Healing
Wound healing rates in the PRP and MSC groups were recorded significantly faster at 21 days post-burn. The wound closure percentage between groups is summarized in
Mean of remaining wound size (in percentage) in each intervention group during weekly observation
|
Intervention |
Initial |
Day 7 |
Day 14 |
Day 21 |
Day 28 |
Day 35 |
|
PRP |
100 |
115.6 |
92.1 |
47.9 |
17.9 |
7.9 |
|
MSCs |
100 |
166.9 |
89 |
50.4 |
21.4 |
11.5 |
|
Control |
100 |
98.7 |
87.5 |
86.4 |
14.5 |
1.3 |
VEGF Expression
The mean VEGF expression in the MSC and PRP groups was 1.97 (p = 0.07) and 1.42 (p = 0.17) times higher than the control group (Figure 2). However, the values were not statistically significant. There was also no statistically significant difference between VEGF expression of the MSC and PRP groups.

Relative mRNA VEGF expression of MSCs and PRP group (ratio to untreated control).
Histologic Analysis
Histological analysis of the burned skin at day 35 showed that all of the groups had developed adequate epithelial lining on the wound surface, dermal connective tissue, and vascular networks. MSCs group displayed the best differentiation outcome since sebaceous glands and fat lobules were also visualized in the tissue sample. These structures were not found in the other two groups (Figure 3).
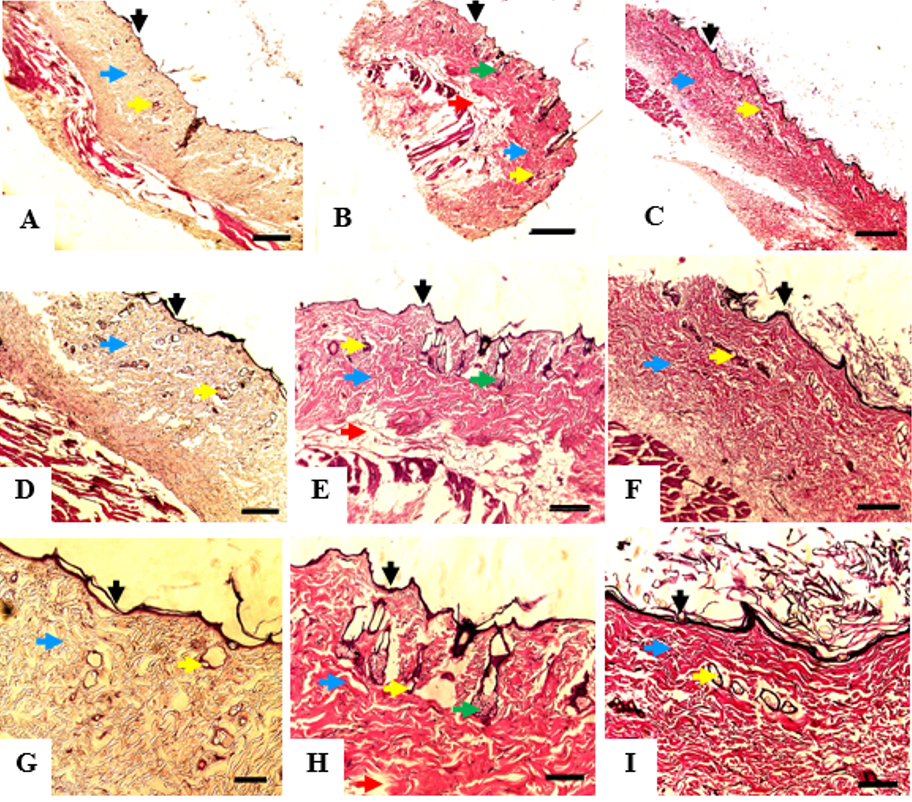
Histologic appearance of the burned skin mice collected after 35 days of treatment; control group (A,D,G), MSCs group (B,E,H); and PRP group (C,F,I). Each specimen was subjected to hematoxylin-eosin (H& E) staining and photographed at a magnification of 40x; Bar = 50 µm (A,B,C), 100x; Bar = 20 µm (D,E,F), and 200x; Bar = 10 µm (G,H,I). Epithelial cells showed in black arrow; blood vessel showed in yellow arrow; connective tissue showed in blue arrow; sebaceous gland showed in green arrow, and fat tissue showed in red arrow.
Discussion
Rats have unique wound healing characteristics compared to human wound healing. In addition to epithelialization, wounds in the rat model have considerable contraction. The wound contraction is caused by the subcutaneous panniculus carnosus muscle and significant collagen formation (fibrosis)43. Wound remodeling phase in rats also begins earlier than in humans- at around 6 days44. The quicker wound healing phase might also explain the fast wound contraction rate in the rat wound model.
Regarding the normal healing time of the rat burn wound model, it mainly depends on the depth of the burn wound. Venter. reported that a deep partial-thickness burn wound requires around 35 days to heal while a full-thickness burn wound might require as long as 49 days to be completely epithelialized45. Their findings were similar to those in our study, where the burn wounds were almost fully healed by day 35. Another interesting finding was increased burn wound size in the PRP and MSC treatment groups during observation on day 7. The increase of burn wound size during the first few days was also reported by Ebrahim . and Hyung Woo Ju .46, 47. A possible explanation for the increase could be the separation of eschar from the surrounding vital tissue, causing the wound edge to retract away from the center of the wound. Further studies are needed to elucidate this finding as other similar studies did not report this occurrence.
PRP contains various cytokines and growth factors, such as VEGF, transforming growth factor (TGF)-β, platelet-derived growth factor (PDGF), and epidermal growth factor (EGF), among others. These cytokines and growth factors promote anti-inflammatory responses, angiogenesis, and epithelialization32. PRP also influences the formation and organization of collagen fibers, reducing the rate of fibrosis28, 34.
MSCs improve wound healing by two main mechanisms which are paracrine effect and direct differentiation2, 18. The paracrine effect of MSCs on wound healing is performed by secretion of various cytokines and growth factors, some of which are similar to those contained in PRP. The role of these cytokines and growth factors are also similar to those of PRP, that is to increase angiogenesis, epithelialization, anti-inflammatory reactions, and anti-fibrotic effects6, 12, 16, 19, 20. MSCs can also migrate and differentiate into skin cells, such as dermal fibroblasts and keratinocytes2. Xenogenic human-derived MSCs have the potential to enhance wound healing in various animal wound models17, 18, 19, 20. Liang Xue even reported that xenogenic MSCs could also differentiate into the epidermis in a mouse wound model19.
Intradermal injection of MSCs and PRP in our study did not seem to shorten epithelialization duration compared to the control group. However, significant wound size reduction was detected earlier on day 21 in these two groups, while it was detected on day 28 in the control group. Previous studies that utilized xenogenic human-derived PRP — either as a single therapy or a combination therapy — showed beneficial effects of PRP on wound healing in animal models48, 49, 50, 51. The discrepancies might be caused by different PRP preparations, research protocols, and animal species.
Some findings of our study are also different from previous studies that have utilized xenogenic human MSCs. In those studies, MSC application accelerated the epithelialization rate significantly17, 18, 19, 20. On the other hand, Hanako Doi. reported similar results to our study. In their study, xenogenic use of umbilical cord blood derived MSCs and Wharton’s jelly derived MSCs did not accelerate wound healing in mice significantly52. In their opinion, the xenogenic application of human MSCs produced immunologic and inflammation responses that negated the favorable effect of the MSCs. However, this explanation contradicted the results of other similar studies, which reported that xenogenic application of MSCs to mice and rats produced anti-inflammatory effects19, 20. In addition, human MSCs have survived in the animal wound environment due to their limited immunogenicity19. These differences could be caused by differences in the protocol and animal models across studies.
In our opinion, there is another possibility that might affect the wound healing duration. The anti-fibrotic effects of MSCs and PRP might attenuate the wound contraction phase of wound healing in rats. As discussed earlier, wound contraction is a unique wound closure mechanism that is found in the rat wound healing process. This hypothesis might explain why the wound healing duration in the PRP and MSC treatment groups showed no significant difference from that of the control group. This theory is supported by the study conducted by Jia Xian Law .48; they studied the role of PRP and tissue-engineered skin substitutes to treat full-thickness wounds in a nude mice model. The study found that the application of PRP with or without tissue-engineered skin substitutes reduced the level of myofibroblasts, which plays an essential role in wound contraction48. Nevertheless, further studies are needed to elaborate on these findings.
Examining the VEGF messenger ribose nucleic acid (mRNA) level in the wound tissue showed an increase of VEGF expression in the MSC and PRP treatment groups compared to the control group. The elevation of VEGF expression in the MSC group is higher than that for the PRP group. Since PRP does not contain living cells, it is explicable that growth factors will decline over time. Jiro Kurita . reported that tissue perfusion improvement was still detected at 4 weeks after PRP treatment23. In our study, the level of VEGF in the PRP group was higher than that of the control group at 5 weeks after xenogenic PRP administration. The capacity of growth factors to persist in the tissue is influenced by concentration of the initial growth factors and the ability of live tissue to store the growth factors23.
Even though the difference between the VEGF levels in the MSC and PRP groups is not statistically significant, this finding might suggest that human-derived MSCs excite a more sustainable angiogenesis effect than PRP. The high level of VEGF expression on day 35 after applying MSCs also implies that human-derived MSCs were most likely to survive in the wound tissue. However, we did not perform any fluorescence labeling and detection techniques.
Histopathological analysis showed that the wound samples that were treated with MSCs had the best differentiation outcome. The MSCs group developed sebaceous glands and fat lobules that were not detected in the other groups. The blood vessels were found more abundantly in the MSC and PRP groups than in the control group. These results correspond well with the level of VEGF expression that was discussed above. The histopathological findings explain that even though human-derived MSCs and PRP did not improve wound healing duration in the rat burn wound model, the quality of the healed tissue is high in these groups. We are aware of some limitations of our study. The macroscopic wound observations was limited to 35 days which hindered the determination of time needed by the wounds to be epithelialized entirely. The confirmation of MSC survival and differentiation ability could not be confirmed definitively due to the lack of fluorescence labeling.
Conclusions
In conclusion, human-derived MSCs and PRP do not accelerate epithelialization duration of burns in the rat burn wound model. However, they improve wound vascularization and cell differentiation. Indeed, MSCs are superior to PRP in enhancing cell differentiation.
Abbreviations
cDNA: Complementary DNA
DMEM: Dulbecco's Modified Eagle's Medium
mRNA: messenger ribose nucleic acid
MSCs: Mesenchymal stem cells
PRP: Platelet-rich plasma
RT-PCR: Real-time polymerase chain reaction
SVF: Stromal vascular fraction
VEGF: Vascular Endothelial Growth Factor
Acknowledgments
None.
Author’s contributions
Conception and design: Karina K, I Rosadi, I Rosliana; Administrative support: Karina K; Provision of study material or patients: Karina K; Collection and assembly of data: JA Biben, K Ekaputri, I Rosadi, I Rosliana, I Afini, T Widyastuti, S Sobariah, WR Subroto; Data analysis and interpretation: all authors; Manuscript writing: Karina K, JA Biben, K Ekaputri, I Rosadi, I Rosliana. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

