
Novel Insights into Genetic Approaches in Glioblastoma Multiforme Therapy
- Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Kelantan 16150, Malaysia
- Department of Neurosciences, Hospital Universiti Sains Malaysia, Kubang Kerian, Kelantan 16150, Malaysia
- School of Industrial Technology, Universiti Sains Malaysia, Penang 11800, Malaysia
- Faculty of applied science, Universiti Teknologi MARA, Shah Alam, Malaysia
- Hospital Seberang Jaya, Jalan Tun Hussein Onn, Seberang Jaya, Permatang Pauh, 13700, Penang, Malaysia
Abstract
Glioblastoma multiforme is one of the most common and malignant types of central nervous system (CNS) tumors. Despite the great advances in treatment modalities and the variety of therapeutic options, it remains largely incurable with continuously growth incidence, due to the genomic instability of glioblastoma multiforme cells, their heterogeneity, and their resistance to chemo- and radiotherapies. The aggressive behavior of these brain tumors and their sequestered location behind the blood–brain barrier restricts the role of the immune system. Great success has been made recently in glioblastoma multiforme treatment using genetic based approaches to selectively target cancerous cells and restore tumor suppressor gene expression or silence specific oncogenes to prevent their expression. The use of genetic approaches has attracted more interest and research and has revealed their ability to regulate the expression of glioblastoma multiforme oncogenes without changing the genotype and thus avoiding possible genotoxicity. This review delivers an overview of glioblastoma multiforme cell biology, tumorigenesis, and immune surveillance, and discusses recent advances in genetic based therapies of glioblastoma multiforme.
Introduction
Glioma is a general term that refers to brain tumors that have been classified based on their presumed cell of origin: astrocytic tumors (including astrocytoma, anaplastic astrocytoma, and glioblastoma), ependymomas, oligodendrogliomas, and mixed gliomas1. Glioblastoma multiforme is considered the most frequently occurring and malignant primary astrocytoma, accounting for more than 60% of all adult brain tumors and approximately 17,000 new diagnoses each year2. Despite the great advances and the variety of modern therapies against glioblastoma multiforme, it is still considered one of the deadliest cancers and is characterized by an extremely poor prognosis3. Its features include high aggressiveness, tremendous invasive capacity, and resistance to conventional therapeutic approaches such as chemo- and radiotherapy4. Gliomas have been classified by the World Health Organization (WHO) according to their level of malignancy into grades I to IV: grade I gliomas include mild lesions that are characterized by low proliferative potential, while grades II to IV are highly invasive and malignant. Glioblastoma multiforme is designated grade IV—the most aggressive, invasive, and undifferentiated type of CNS tumor5. Mounting knowledge of the properties and characteristics of glioblastoma multiforme, being the most aggressive and located in very sensitive part of the body, have sparked the recent application of advanced gene-based therapies to target the cancer’s molecular mechanisms.
Gene therapy has been defined by many authors as the introduction, alteration, or removal of certain nucleic acids—such as genes, oligonucleotides, gene segments, microRNAs (miRNAs), or small interfering RNA (siRNA)—from targeted cells, leading to altered gene expression and/or the synthesis of an exogenous protein6, 7. A variety of gene therapy approaches have been developed and tested for use in glioblastoma multiforme therapy, especially in advanced cases; many of these approaches show promising potential and provide hope for a new generation of molecular therapies. The first attempt at using gene therapy to treat glioblastoma was published in 1996 and involved the use of a viral vector for gene delivery to the patient8. Since then, various viral and non-viral vectors have been used to deliver the genes to glioblastoma tumors9. Epigenetics is considered a marker of human cancers and represents any mitotically heritable alteration in gene expression apart from alteration of the DNA sequence (mutations)10, 11. In the present review, we comprehensively discuss the natural biology, metastasis, immune surveillance, and current therapeutic approaches to glioblastoma multiforme. We also critically evaluate the recent advances in genetic based therapies, including oncogene silencing, suicide gene therapy, targeting angiogenesis, immunization gene therapy, targeting tumor cell-derived exosomes, tumor gene repair, and whole-genome editing therapies.
Glioblastoma multiforme tumorigenesis and immune surveillance
The past few decades have produced significant advances in the understanding of the nature of cancer cell biology, especially for glioblastoma multiforme. The dominant oncogenes and tumor suppressor genes that control the respective activation, upregulation, or inhibition of cellular functions impart aberrant characteristics onto normal cells, leading to their transformation into malignant cells12. Glioblastoma is classified as class IV, the most aggressive, invasive, and undifferentiated type of CNS tumor5. However, the manifest the disease was reported to be associated with cancer cells, in addition to conscript and corrupt resident, which recruited normal cells to serve as contributing members around the cancer cells13. The tumor microenvironment was reported to play a critical role in cancer metastasis and malignancy; the interaction between neoplastic tumor cells and their surrounding stroma leads to chronic proliferation and formation of organ-like structures, which are known as tumors and typify most human cancers14, 15. The multifactorial contributions of activated or recruited brain cells to glioblastoma multiforme may be performed by some or all of the factors presented in Figure 1, including evading growth suppressor mechanisms of cells, chronic proliferative signaling, angiogenesis, resisting cell death, activating invasion and metastasis, enabling replicative immortality, evading immune-mediated destruction, and reprogramming energy metabolism16.
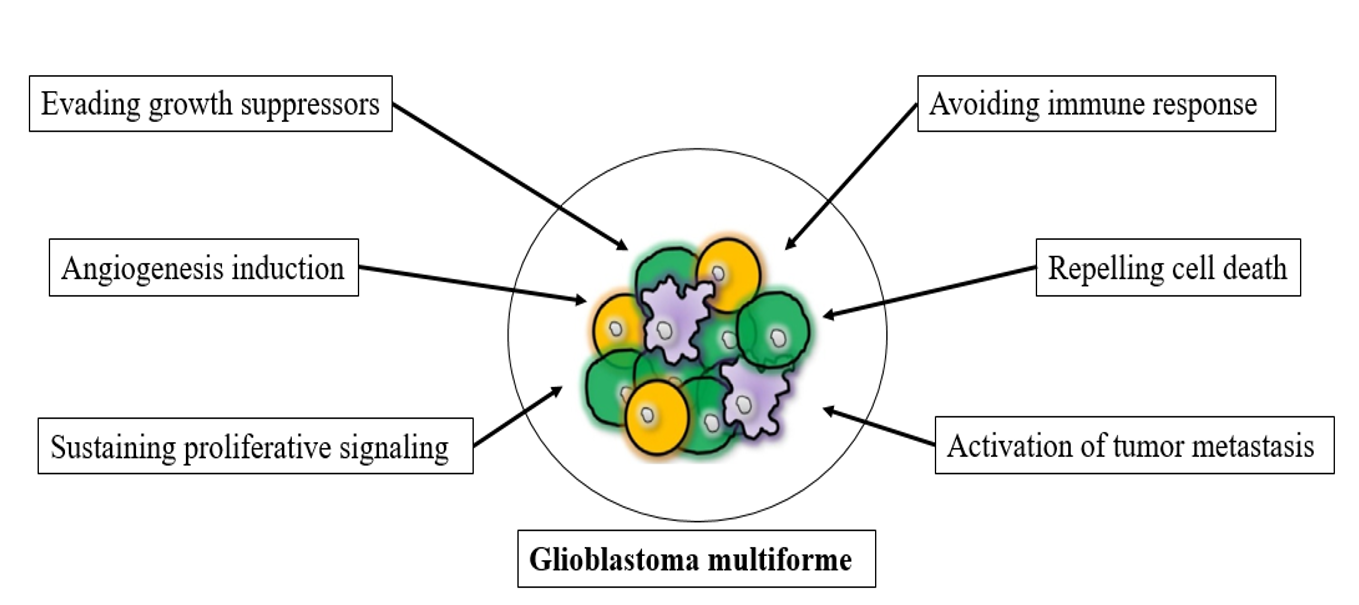
Illustration of multifactorial contributions of activated or recruited brain cells to Glioblastoma multiforme.
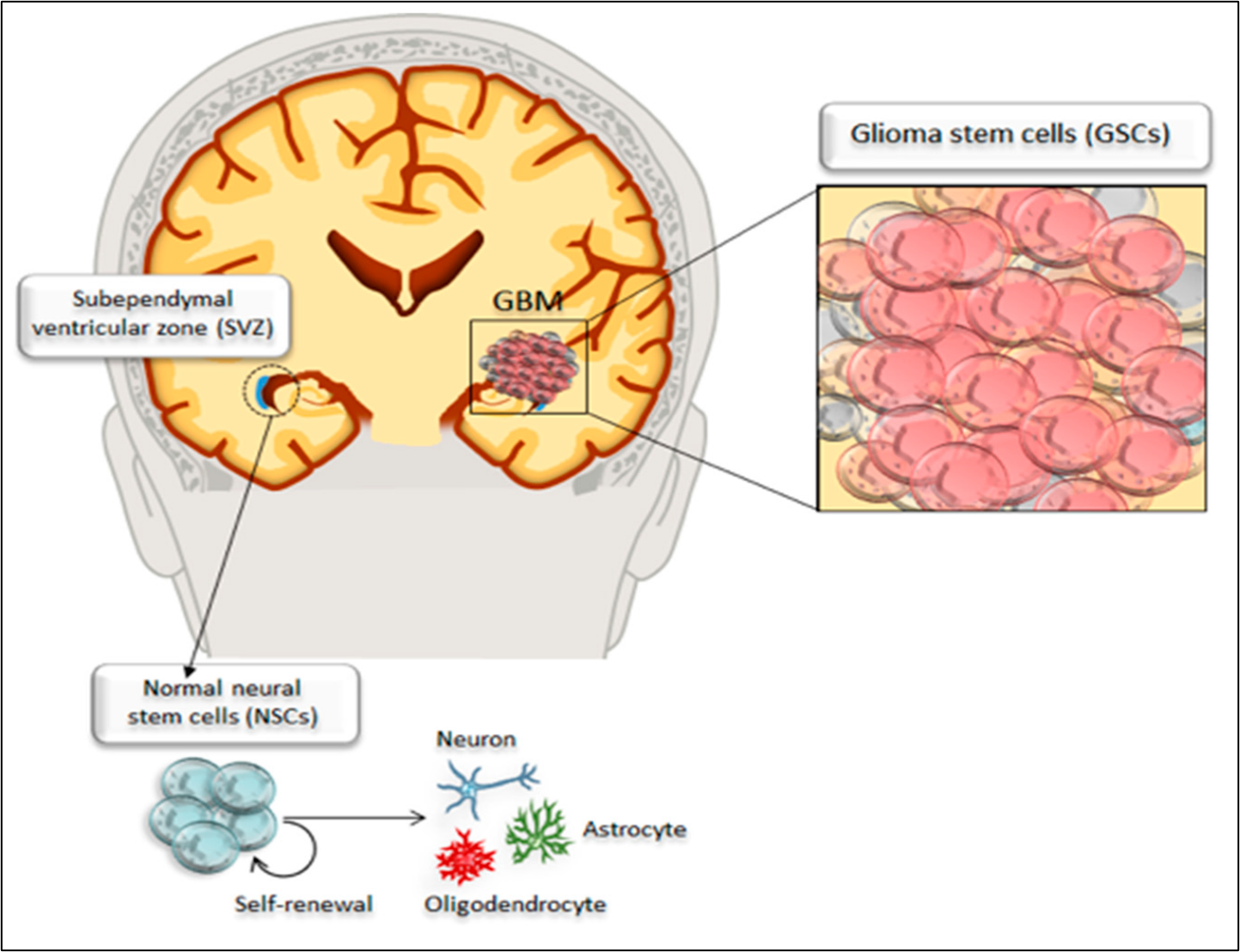
Schematic drawing of tumorigenesis of glioblastoma multiforme (GBM) from cancer stem cells in glioblastoma. Adapted from Ryskalin
Glioblastoma multiforme cell biology
The most frequent locations for glioblastoma are the cerebral hemispheres; as reported by Nakada .18 more than 95% of glioblastoma multiforme tumors arise in supratentorial regions and only a small percentage occur in the spinal cord, cerebellum, and brainstem. Transformation of a normal cell into a cancerous cell may occur due to exposure to a carcinogenic agent, resulting in cells gaining the properties of rapid proliferation and metastasis9, 19. Many studies have focused on the metabolism of different cancer cells and have revealed that metabolic reprogramming is a unique hallmark of cancer cells20, 21. Stone & Darlington22 identified a few genetic changes that occurred within a single cell that were able to increase its proliferation, motility, migration, extracellular matrix metabolism, and tissue penetration. Macroscopically, glioblastoma multiforme is highly heterogeneous, featuring multifocal necrosis, hemorrhage, and cystic and gelatinous areas1. The glioblastoma multiforme tumor develops in the white matter of the CNS and is usually represented by a single, large, irregularly shaped lesion that resembles an anaplastic astrocytoma23. Brain cancer stem cells share core biological characteristics with normal stem cells, such as self-renewing potential and maintenance of proliferation. In particular, glioblastoma multiforme contains a subpopulation of cancer stem cells with enhanced self-renewal ability24. This is the case in brain cancer stem cells that reside in the dentate gyrus of the hippocampus and perivascular niches within the subependymal ventricular zone25; these cells have been reported to give rise to extremely highly proliferative tumor cells compared with other types of cancer cells, constituting an intense tumorigenic bulk within the parenchymal cells of the healthy brain as shown in Figure 217. To date, it is unclear whether glioma stem cells originate from normal neural stem cells or from undifferentiated neural or glial cells transformed into glioma stem cells; in any case, glioma stem cells are considered the main drivers of neoplastic transformation26, 27.
Metastasis and immune surveillance of glioblastoma multiforme
The genomic instability of glioblastoma cells, their heterogeneity, behavior, infiltrative capacity, and sequestered location were found to restrict the role of the immune system against the tumor cells. The immune cells are not able to reach the site of tumor due to the presence of the blood–brain barrier, which restricts their action28. Furthermore, glioblastoma cells are able to develop several mechanisms to evade immune surveillance, including the release of inflammasome-dependent cytokines and the direct or indirect release of other costimulatory molecules that shape an immunosuppressive tumor microenvironment during the progression of the cancers29, 30. Formation of new capillaries and blood vessels around the growing tumor is known as angiogenesis and was reported to be vital process highly observed within glioblastoma multiforme tumors—from small and localized tumors to enlarging ones—allowing them to metastasize31. The environment of distant metastatic target organs usually undergoes reprogramming to favor and enhance the growth and spread of the tumor32, 33. Tumor cell invasion and migration (tumor metastasis) are key events in the metastatic cascade; Lambert .34 studied different mechanisms of tumor metastasis and revealed that several cytokines are essential to this process, including IL-18 and IL-1β, which are the two most widely studied mediators of cancer cell invasion and migration. These cytokines facilitate the invasion and spread of glioblastoma multiforme cells. Other investigations have reported that cancer cells may also circulate as dormant tumors and become clinically apparent within their lifetime35, 36.
Therapeutic approaches of glioblastoma multiforme
The genetic instability of glioblastoma, its heterogeneity, behavior, infiltrative capacity, and sequestered location make it one of the most challenging cancers for conventional treatment techniques28. A variety of therapeutic approaches have been developed to treat glioblastoma multiforme and differ in terms of targeting either the tumor cells or enhancing the anti-cancer immune response; many factors influence the choice for the most suitable option in each cancer patient, which can be influenced by either tumor-associated factors or patient- and physician-associated factors. However, the ultimate goal of all treatments is to eradicate the glioblastoma multiforme cells, minimize the sequelae of treatment, preserve or restore form and function, and prevent any potential subsequent primary cancers37, 38, 39.
Summary of the advantages and disadvantages of glioblastoma multiforme therapeutic options
|
Therapeutic option |
Advantages |
Disadvantages |
Ref |
|---|---|---|---|
|
Surgery |
Straightforward and does not require advanced equipment’s |
Very difficult to operate, cannot restrict the cancer alone, unsuitable for advanced tumors and must be combined with other salvage therapy |
|
|
Chemotherapy |
Salvage therapy for glioblastoma multiforme after surgery |
Highly toxic to normal cells, glioblastoma cells are able to overtake chemo-treatment without major damage |
|
|
Radiation therapy |
Salvage therapy to improve treatment outcome and reduce its duration |
Not suitable for metastatic and advanced glioblastoma, possibly increase the accumulation of gadolinium in the brain and radio resistance |
|
|
Virotherapy |
Selective cytotoxicity to cancer cells, stimulate anti-tumor immune responses |
Potential pathogenicity in some viruses, blood brain barrier may limitate viral accessability to glioblastoma cells and the potential resistance |
|
|
Immunotherapy |
Self-improving response, induction of long-term immunity to glioblastoma |
Developing of immunosuppressive environment by glioblastoma cells to limitate the immune action and potential of autoimmune induction |
|
|
Gene therapy |
Fast and limited dosage onset, highly specific to cancer gene and safe for normal genes |
Depending on the strategy; possible gene alteration, which may lead to other defects, still under developing and experiments |
|
|
Epigenetic therapy |
Does not cause any gene alteration and selectively targeted defected tissue |
Extremely expensive, and still under investigation |
|

Major gene based strategies for glioblastoma multiforme treatment.
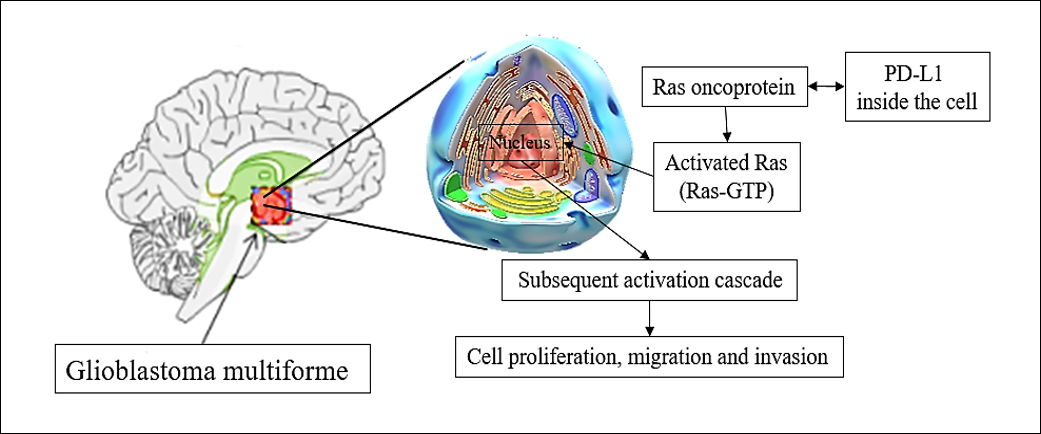
Illustration of PD-L1 gene role in the proliferation, migration and invasion of glioblastoma cells.
Genetic based approaches for glioblastoma multiforme therapy
Gene editing allows for selective targeting of cancerous cells by restoring tumor suppressor gene expression or silencing specific oncogenes and preventing their expression47. Most current gene therapy approaches aim at the altering, inserting, removing, or modulating particular genes instead of generalized, whole-genome editing48, 49. Figure 3 presents the most common approaches of glioblastoma multiforme gene therapy discussed below.
Oncogene silencing
Oncogene silencing consists of the targeted delivery of a particular nucleic acid into tumor cells, which leads to downregulation of specific genes (silencing)50. The extracellular matrix adhesion of glioblastoma multiforme cells is an important step for their invasion and many proteins have been investigated to play critical roles in accelerating this invasion51. The expression of these proteins has been reported to be extremely high in glioblastoma multiforme patients; therefore, silencing of oncogenes that express these proteins would inhibit the spread of glioblastoma multiforme cells51, 52. is a gene known for its oncogenic role in glioblastoma multiforme as a guanine-nucleotide exchange factor for . It has been reported that is highly upregulated in human glioblastoma and other brain tumors53. Fan .54 evealed that downregulation of inhibited glioblastoma cell proliferation and metastasis and induced autophagy of the cancer cells. is an oncoprotein that has been reported to play a significant role in glioblastoma oncogenesis, and compelling evidence has demonstrated its role in the regulation of tumor cells as well as the migration and invasion of tumor cells under normal nutrient conditions55. In a recent study, Luo .56 silenced the expression of and observed marked reduction in adhesion of glioblastoma cells. The authors reported that significantly contributed to the adhesion of glioblastoma cells by regulating the lysosomal degradation of the protein integrin subunit beta 1 () under serum starvation, and its silencing led to a reduction in the adhesion of glioblastoma cells and the levels of ITGB1 protein. Zinc finger E-box-binding homeobox 2 () is a protein-coding gene that plays a critical role in the transcriptional regulation of various cellular functions and is abnormally expressed in brain tumors such as glioblastoma multiforme57. Safaee .58 investigated the effect of silencing on the cell cycle, apoptosis, and cytotoxicity of different glioblastoma cell lines and revealed that the suppression of induced apoptosis in all tested cell lines. Furthermore, the authors observed cytotoxic effects and marked reduction in migration of glioblastoma cell lines, suggesting the promising potential of silencing for the treatment of glioblastoma multiforme. Targeting programmed death ligand-1 () or programmed cell death protein 1 () is another revolutionary strategy that has been applied to glioblastoma multiforme treatment. Qiu .59 studied the role of in glioblastoma multiforme patients and reported that it bound to the Ras oncoprotein in glioblastoma cells which altered gene expression and led to significant acceleration of cellular growth, migration, and invasion pathways. The same authors silenced and revealed that it played a vital role in glioblastoma multiforme cell proliferation and migration. The overexpression of promoted glioblastoma multiforme cell development and invasion in rodents, while its silencing abolished them. Figure 4 presents a summary of the role of in glioblastoma proliferation, migration, and invasion.
Suicide gene therapy
Suicide gene-based therapy consists of inserting genes that encode for cytotoxic proteins into glioblastoma cells either directly by inserting the toxin gene to reduce the viability of the cancer cells or indirectly by introducing a gene encoding an enzyme or protein with cytotoxic effects60, 61. Different strategies for suicidal gene delivery have been evaluated; Li .62 developed a novel therapy for glioblastoma based on mesenchymal stem cells to counteract the aggressive spread and dissemination of the cancer. The authors genetically engineered the mesenchymal stem cells and integrated the suicide gene into their nuclei. Upregulation of was clearly observed in the patients’ glioblastoma cells, in contrast to their non-neoplastic cortex cells, suggesting the high selectivity of this therapeutic approach. The same authors monitored the suicide gene products and demonstrated a significant improvement in the clinical efficacy, suggesting great potential for this approach in complicated brain cancers.
Gene therapy of glioblastoma with suicide genes using viral vectors has been also studied. The herpes simplex virus thymidine kinase () gene was inserted into glioblastoma cells using a viral vector; the expression of the gene led to the production of protein, which phosphorylated and transformed the non-toxic prodrug ganciclovir into toxic ganciclovir-triphosphate63. Toxic ganciclovir-triphosphate produced from transfected cells was transported into neighboring cancer cells through gap junctions and led to a widespread and strong antitumor effect, even in cells that had not been genetically modified. Park .64 used the same method and combined the thymidine kinase gene with curcumin; the combined delivery of both the thymidine kinase gene and curcumin had higher therapeutic effects on glioblastoma than either treatment alone.
Targeting angiogenesis
Uncontrolled and rapid growth of tumor cells leads to hypoxia and subsequent secretion of cellular angiogenesis signals such as angiopoietins, IL-8, fibroblast growth factor-2, or vascular endothelial growth factor to secure the oxygen and nutrient supply to the tumor cell, a vital process for tumor progression65, 66. Two major strategies have been pursued to target glioblastoma cell angiogenesis: upregulating the expression of anti-angiogenic factors and downregulating the expression of pro-angiogenic factors60. Sousa .67 developed an anti-angiogenic monoclonal antibody for glioblastoma multiforme treatment in animal models using bevacizumab-loaded poly(D,L-lactic-co-glycolic acid) nanoparticles to decrease off-target organ toxicity and to circumvent the blood–brain barrier. The monoclonal antibodies were administrated intranasally and showed high brain bioavailability 7 days after administration. The authors reported significant reduction in tumor growth 14 days after administration which resulted from the high anti-angiogenic effect. Some studies have suggested that resistance to anti-angiogenic therapy may develop as a result of immune activation68, 69, specifically as a result of the action of pro-angiogenic M2-polarized macrophages. Some researchers have aimed to reprogram or inhibit the M2 phenotype to prevent the development of resistance to anti-angiogenic therapy and have revealed the phenotype to augment strong anti-angiogenic activity in animal models70, 71, 72. Glioblastomas are characterized by the release of vascular endothelial growth factor, which acts as a regulator and promoter of angiogenesis. Some anti-angiogenic therapies target this regulator or its receptor to inhibit the growth of glioblastoma cells, using monoclonal antibodies against vascular endothelial growth factor in addition to tyrosine kinase inhibitors that target the receptors of vascular endothelial growth factor73, 74.
Targeting tumor cell-derived exosomes
Exosomes are a type of extracellular nanovesicle that have an important role in intercellular communications and consist of biological macromolecules such as proteins, DNA, and/or RNA75. The exact content of exosomes is dependent of the type of cell from which they are derived and its physiological condition76, 77. Importantly, many studies have revealed that after internalization by a secondary cell, the released exosomes induced significant phenotypic alterations depending on their content78, 79. Tumor cells generally secrete higher quantities of exosomes compared with normal cells; these secretions have been reported to play a vital role in promoting tumor progression by inducing malignant transformation of normal cells, cancer-associated fibroblast transformation, tumor escape from the immune system, angiogenesis, and metastasis80. Glioblastoma stem cells have been used to release exosomes containing microRNAs to mediate cellular communication; Tian .81investigated whether glioblastoma stem cell-derived exosomes that contained the microRNA could influence angiogenesis in microvessel endothelial cells in glioblastoma. The authors reported that, in glioblastoma, the tumor suppressor gene was downregulated while was upregulated; this finding was explained by the activation of the signaling pathway by , which led to downregulation. Furthermore, the authors reported that glioblastoma stem cell-derived exosomes containing promoted the proliferation, migration, tube formation, and angiogenesis of tumor cells, suggesting that targeting of these exosomes could represent a potential therapeutic approach. Domenis .82investigated the properties of immune-mediated tumor-derived exosome release upon activation of toll-like receptor 4 and found that treatment of the tumor influenced tumor-derived exosome composition and boosted its immunosuppressive ability, suggesting that the activation of toll-like receptor 4 supports tumor progression by stimulating the excretion of more effective immunosuppressive exosomes from the cells. This excretion of exosomes allows cancer cells to escape immune surveillance. Hypoxia-mediated stress in glioblastoma multiforme produced qualitative and quantitative changes in exosome content, with significant elevation of protein-lysine 6-oxidase, vascular-derived endothelial factor, and thrombospondin-1, which were all associated with tumor progression, metastasis, and angiogenesis83. Kore .83demonstrated that hypoxia-related exosomes induced significant differential gene expression in recipient glioblastoma cells, showing a marked upregulation of small nucleolar RNA, transcript, among others, and significant downregulation of voltage-gated potassium channels. Glioblastoma cell-derived exosomes are potential novel therapeutic targets. A significant number of publications address the qualitative and quantitative changes in these nanovesicles and their role in tumor development, and promote the development of preclinical and clinical trials involving these potential new targets84, 85, 86.
Immunization gene therapy
Immunization gene therapy is a broad therapeutic approach that involves enhancing the efficacy of the patient’s immune system against glioblastoma multiforme cells using chimeric antigen receptor T (CAR-T) cell therapy, tumor vaccine therapy, or cytokine gene therapy87. In a recent study, Agliardi .88 used genetically engineered CAR-T cells targeting receptor variant III of the glioblastoma tumor-specific epidermal growth factor in a mouse model and revealed that the engineered cells alone were unable to fully control the established tumors; however, the authors achieved lasting antitumor response when they combined single and locally delivered doses of IL-12. The cytotoxicity of CAR-T cells was significantly enhanced in the presence of IL-12, which also reshaped the tumor microenvironment, decreased the number of regulatory T cells, and increased the infiltration rate of pro-inflammatory CD4 T cells89. In a similar study, Tang .90 transduced a CAR-targeting B7-H3 transmembrane protein into T cells using a lentivirus. The authors assessed the antitumor effects of the transmembrane protein B7-H3-specific CAR-T cells in vitro and in vivo with primary and glioblastoma cell lines and revealed that expression levels were highly correlated with malignancy grade; in addition, most of the clinical glioma samples were positive for . The study also revealed the association of with poor survival rates in both low-grade glioma and glioblastoma patients. The results of cytotoxic and ELISA assays confirmed the specific antitumor effects of the engineered CAR-T cells on both cell lines. The authors added that the median survival time of the CAR-T-cell-treated group in the orthotropic glioblastoma models was significantly longer than that of the control group, suggesting promising potential for targeting CAR-T in glioblastoma multiforme treatment.
Tumor vaccination has also been studied in glioblastoma multiforme and involves presenting the tumor-specific antigens to the immune system and triggering an immune response against the antigens91. Dendritic cell-based vaccines are one of the novel strategies being tested in recent clinical trials that mediate anticancer immune reactions92. A number of dendritic cell-based vaccines for glioblastoma multiforme have been developed and are currently undergoing clinical investigation93. Erhart .94 found that glioblastoma multiforme patients with pre-existing antitumor characteristics seemed to survive longer under dendritic cell-based immunotherapy (Audencel) than patients who lacked these characteristics. The authors reported the inability of Audencel therapy to induce a significant clinical response, but the treatment had a strong effect on the immune system. The pre-vaccination blood count of CD8 T lymphocytes and the production capacity of the enzyme-linked immunosorbent spot granzyme B upon exposure to the tumor antigen were significantly correlated with overall patient survival. Dendritic cell-based immunotherapy for glioblastoma multiforme led to significant upregulation of many cytokines promoting Th1 activation, better antitumor response, higher post-vaccination levels of IFNγ; in addition, the levels of CD8cells in the patients’ blood were indicative of better prognosis compared with unvaccinated patients94. Other studies proposed immune checkpoint inhibitors for the enhancement of glioblastoma multiforme treatment95, 96, 97. Current efforts focus on the combination of immune checkpoint inhibitors with different glioblastoma treatment modalities such as and checkpoint inhibitors98, 99. Khaddour .100 reviewed three novel techniques involving immune checkpoint inhibitors, including the administration of adjuvant immune checkpoint inhibitors following surgical resection of glioblastoma, patient selection for treatment with immune checkpoint inhibitors, and combining immune checkpoint inhibitors with other novel therapies (Figure 5). Recently, a variety of receptors, molecules, and pathways have been investigated and have emerged as potential targets for combined immune checkpoint inhibitors, such as TGF-β inhibitors, CD47 blockade, and colony-stimulating factor-1 ligand inhibitors101. Rationally designed immunization gene therapy and combinatorial approaches offer promising treatments for glioblastoma multiforme.

Proposed therapeutic approaches of using immune checkpoint inhibitors for improving glioblastoma multiforme treatment. A: following the surgical resection of glioblastoma with adjuvant immune checkpoint inhibitors administration, B: patient selection for treatment with immune checkpoint inhibitors and C: combination of immune checkpoint inhibitors with other novel therapies. Adapted from Khaddour
Tumor gene repair
Abnormal expression of different DNA repair genes is frequently associated with tumorigenesis, although the role of these repair genes in the progression and development of glioblastoma remains unclear102. Repairing the damage in tumor genes involves induction of limited, selective alterations to the genetic material of tumor cells, which is consider an advantage of this approach; however, this process contributes to the development of resistance to genotoxic therapies based on tumor-driving cells103. Struve .104 observed significantly elevated expression of several DNA mismatch repair proteins in epidermal growth factor receptor variant III cells and samples from glioblastoma patients; this expression was most pronounced for DNA mismatch repair protein 2 and 6. Epidermal growth factor receptor variant III-specific knockdown reduced mismatch repair protein expression, thereby increasing resistance to the alkylating agent temozolomide and demonstrating that the oncoprotein epidermal growth factor receptor variant III sensitizes a fraction of glioblastoma through the upregulation of DNA mismatch repair proteins. In separate study, Lin .105revealed that the inhibition of RNA-binding protein Musashi-1 in glioblastoma multiforme patients radiosensitized tumors, prevented cancer stem cell selection in radiotherapy, and reduced tumor invasion. The authors reported that Musashi-1 enhanced tumor invasion via vascular cell adhesion protein 1 and modulated glioblastoma multiforme radioresistance via hyperactivation of the DNA damage response process through evasion of apoptosis and increased homologous recombination repair. Therefore, knockdown of Musashi-1 induced the accumulation of DNA damage in irradiated glioblastoma multiforme cells and promoted their depletion in vitro. Kun .102performed clustering to screen for potentially abnormal DNA repair genes associated with glioblastoma multiforme prognosis and revealed that five DNA repair genes (, , , , and ) were significantly related to glioblastoma multiforme prognosis. The prognosis of glioblastoma multiforme can be predicted from the expression of DNA repair genes, which may also represent future therapeutic targets.
Genome editing
Genome editing-based therapy consists of the modification of whole-body intracellular DNA in a sequence-specific manner via substitution, insertion, deletion, or integration106. Maeder .107 reviewed the most common nucleases used for genome editing, including transcription activator-like effector nucleases, zinc finger nucleases, meganucleases, and the CRISPR/Cas9 system. Among these strategies, CRISPR nuclease Cas9 is the most advanced and versatile system of gene editing technology; it is characterized by highly specific recognition of its target chromosomal DNA and eventual gene disruption108, 109. This technology opens multiple avenues for the treatment of cancers such as glioblastoma multiforme. Rosenblum .108utilized CRISPR-encapsulating lipid nanoparticles to promote therapeutic gene editing in vitro through the disruption of key glioblastoma multiforme survival genes in murine and human glioblastoma cell lines and in an aggressive syngeneic glioblastoma mouse model. The authors revealed that a CRISPR lipid nanoparticle-based platform could potentially be developed and utilized in human clinical trials as a new therapeutic modality for glioblastoma multiforme. To test and confirm the role of the CRISPR/Cas9 system in the induction of significant gene mutations in animal genome atlases of glioblastoma multiforme, Chow .110analyzed a cloned library into an adeno-associated virus vector encoding sgRNA that targeted Trp53 and astrocyte-specific GFAP-Cre (frequently mutated in glioblastoma multiforme). The authors reported that, after using the adeno-associated virus vector-based CRISPR/Cas9, many genes were significantly mutated, suggesting that the CRISPR/Cas9 system could play a critical role in glioblastoma multiforme treatment. In another recent investigation, MacLeod.111used the CRISPR-Cas9 system in patient-derived glioblastoma multiforme stem cells to elucidate the function of the coding genome. The authors identified actionable pathways responsible for the proliferation of cells and revealed the gene-essential circuitry of glioblastoma stemness and growth. The authors also revealed the mechanisms of temozolomide resistance in glioblastoma cells that could lead to combination strategies.
Conclusion
Glioblastoma multiforme is often a fatal disease and most conventional treatment approaches available to patients are only minimally effective. In this review, we discussed the most recent advances in genetic based therapeutic approaches for glioblastoma multiforme. Significant advances have been made in the past decade in developing novel therapeutic approaches using genetic strategies. These advanced techniques have the potential to regulate the expression of glioblastoma multiforme oncogenes without altering the genotype and thus avoid possible genotoxicity. Despite the paucity and expense related to such research, future therapies for chronic and sensitive diseases such as glioblastoma multiforme could involve genetic and/or epigenetic approaches.
Abbreviations
CAR-T: chimeric antigen receptor T; CNS: The central nervous system; CRISPR: Clustered regularly interspaced short palindromic repeats; GBM: Glioblastoma multiforme; HSVtk: The herpes simplex virus thymidine kinase; IL: Interleukin; miRNAs: MicroRNAs ; PD-1: Programmed cell death protein 1; siRNA: Small interfering RNA; WHO: World Health Organization
Acknowledgments
The authors would like to thank the collaboration between Universiti Teknologi MARA, Shah Alam, Malaysia, Universiti Sains Malaysia, Penang, Malaysia and Hospital Seberang Jaya that made this work possible.
Author’s contributions
All authors contributed equally to this work. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

