
Biochemical disorders in the thyroid gland in rats with hyperhomocysteinemia
- Educational and Scientific Centre “Institute of Biology and Medicine” Taras Shevchenko National University of Kyiv, Volodymyrska 64/13, 01601, Kyiv, Ukraine
- Bogomolets National Medical University, Tarasa Shevchenko Blvd, 13, 01601, Kyiv, Ukraine
- National Pirogov Memorial Medical University of Vinnytsya, Pirogova 56, 21018, Vinnytsya, Ukraine
Abstract
Background: In recent years, significant progress has been made in elucidating the mechanisms of development of complications associated with hyperhomocysteinemia (HC), but little is known about the disorders in the thyroid gland. This study aimed to determine the levels of low-molecularweight degradation products and peptides in the thyroid gland of rats with HC. In addition, markers of inflammation and the proteolytic state were also assessed.
Methods: HC was induced by intragastric administration of DL-homocysteine thiolactone (100 mg.kg-1 of body weight) to albino non-linear male rats. The levels of low-molecular-weight substances (LMWS) of various nature were detected at wavelengths of 254 and 280 nm. The levels of MMP-2 and cytokine profiles were detected by enzyme-linked immunosorbent assay. The protein composition and the presence of active enzymes were assessed by SDS-polyacrylamide gel electrophoresis and zymography, respectively.
Results: Obtained results can indicate the enhancement of catabolic reactions, confirmed by the accumulation of LMWS in the blood and thyroid gland of the rats with HC. The state of inflammation, evidenced by the significantly increased pro-inflammatory cytokines, was also revealed. HC was accompanied by the activation of proteolysis, expressed by an increase in overall proteolytic activity, the level of MMP-2, and changes in protein profile. Proteins accumulated with a molecular weight of less than 30 kDa simultaneously with the decrease in the level of high-molecular-weight proteins.
Introduction
The pathogenesis of many diseases, especially those associated with prolonged oxidative stress or persistent inflammation, is often accompanied by an increase in catabolic reactions, leading to the formation of low-molecular-weight degradation products1, 2, 3. The accumulation of low-molecular-weight substances (LMWS) in tissues and biological fluids can be considered a nonspecific marker of increased catabolism4. Due to the structural heterogeneity, LMWS exert various biological activities and can influence metabolism through direct action on proteins, lipids, and nucleic acids. Furthermore, being structurally similar to some regulatory molecules, part of LMWS can bind to cell receptors affecting intracellular processes. Usually, the negative impact of LMWS is complex, manifests itself at various cellular levels, and can lead to damage to molecules, alteration in the integrity of cell membranes, and disruption of intracellular signaling and cell communications. The literature shows a link between the elevation of circulating homocysteine and the development of inflammation5. On the other hand, pro-inflammatory cytokines increase the risk of pathologies (acute ischemic stroke, myocardial infarction) that are characterized by changes in the concentration of homocysteine6, 7, 8. Moreover, inflammation is closely associated with the activation of proteolytic enzymes, which may be a factor leading to the accumulation of peptide components of LMWS.
Hyperhomocysteinemia is known to be a complex and heterogeneous disease that affects all systems, resulting in the development of serious complications. Despite the progress in understanding the pathogenetic mechanisms of HC, there is a lack of information on HC-mediated disorders in the thyroid gland. Considering the significant role of thyroid hormones in maintaining the metabolic status of the whole organism, the study of the causes leading to thyroid dysfunction can help prevent a decrease in the functional activity of the organ in HC9, 10. This study aimed to assess the level of LMWS in the thyroid gland of HC rats, which can be an indirect indicator of catabolic activity in the organ. A cytokine profile and proteolytic activity were also investigated to clarify the involvement of inflammation and proteolysis in the development of HC-related disorders.
Methods
Reagents
Thiolactone DL-homocysteine, tris(hydroxymethyl)aminomethane, o-phenylenediamine, hydrogen peroxide, sodium dodecyl sulfate, Coomassie Blue R-250, gelatin, acrylamide, and N,N'-methylenebisacrylamide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Monoclonal antibodies anti-INFγ, anti-IL-1β, anti-IL4, anti-IL-10, anti-IL-12, and anti-MMP-2 were purchased from Santa Cruz Biotechnology, Inc. (Dallas, Texas, USA). Horseradish peroxidase-conjugated secondary antibodies were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and reagents used in this study were of analytical grade quality and available commercially.
Animals and experimental design
A total of 60 albino non-linear male rats were used in the study. All experiments on animals were performed in compliance with the international principles of the European Convention to protect vertebrate animals used for experimental and other scientific purposes (Strasbourg, 1986). The Ethical Committee approved the study of the Taras Shevchenko National University of Kyiv (protocol №3 approved 25.11.2020). The experiments were started after seven days of animal acclimation in the animal facility of the Taras Shevchenko National University of Kyiv, maintained under constant conditions of temperature (22 ± 3°C), humidity (60 ± 5%), and light (12 h light/12 h dark cycle). Standard rodent food and water were provided . Animals of different ages were used in the current study: one-month-old rats (90 - 100 g) corresponded to young animals; six-month-old rats (190 - 230 g) corresponded to adult animals, and twenty-month-old rats (290 - 320 g) corresponded to old animals.
Hyperhomocysteinemia was induced by intragastric administration of DL-homocysteine thiolactone (Sigma-Aldrich, USA) diluted in 1 % starch solution (100 mg·kg of body weight). DL-homocysteine thiolactone was administered using a nelaton catheter (size 6) once daily for 28 days11. The control rats received an equal volume of 1 % starch. Thus, there were 6 experimental groups with 10 animals in each: 1) Control_young, 2) HC_young, 3) Control_adult, 4) HC_adult, 5) Control_old, and 6) HC_old. HC development was confirmed by the high blood level of homocysteine (above 15 µmol·L). The level of homocysteine in blood plasma was determined by enzyme-linked immunosorbent assay using the kit «Homocysteine EIA» (Axis-Shield, UK). On the 29 day since the start of the experiment, animals were sacrificed. The animals of the groups Control_young and HC_young were killed by cervical dislocation. The animals of the groups Control_adult, HC_adult, Control_old, and HC_old, were killed by decapitation.
Thyroid gland sample preparation
To obtain 10% homogenate of the thyroid gland, 1 g of tissue was homogenized in 9 ml of ice-cold 50 mM Tris-HCl buffer pH 7.4 and centrifuged at 12,000 g for 30 min at +4°C. The supernatant was collected and used for further studies. The Bradford method determined the protein concentration using crystalline bovine serum albumin as a standard12.
Determination of MMP-2 and cytokine levels
The levels of MMP-2 and cytokines were estimated by enzyme-linked immunosorbent assay (ELISA) according to the standard instructions13. ELISA plates were coated overnight at +4°C with samples of thyroid gland previously diluted with Tris–HCl buffer pH 7.4 to a protein concentration of 10 μg·mL. The next day, the plates were washed and blocked with 5% nonfat dry milk for 1 h at 37 °C. After that, plates were incubated for 1 h at +37 °C with the primary antibodies against IFN-g, IL-1β, IL-12, IL-4, IL-10, and MMP-2. Next, plates were washed and incubated for 1 h at +37 °C with the secondary antibodies conjugated to horseradish peroxidase. After washing, substrates (o-phenylenediamine and hydrogen peroxide) were added. The addition of 2.5 N H2SO4 stopped the reaction. The absorbance was read at 492 nm by a microplate reader (mQuant™, BioTek Instruments Inc, USA).
Determination of the level of low-molecular-weight substances
The fraction of LMWS was isolated according to the method described by Nikolajchik . (1991)14. First, the supernatant was mixed with 1.2 M HClO at a 1:1 (v/v) ratio to precipitate the proteins. Then, after centrifugation at 10 000 g for 20 min at 4°C, the supernatant was neutralized by 5 M KOH to pH 7.0, and the sample was subjected to the centrifugation step again. After ethanol was added to the final concentration of 80%, the samples were kept at 4°C for 30 min and centrifuged. The optical density of the supernatants was determined with a spectrophotometer Smart SpecTMPlus (BioRad, USA) at 210 nm, 254, and 280 nm. The level of LMWS was expressed as rel. units per g of thyroid gland tissue.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Zymography
SDS-PAGE was carried out using 4% (w/v) stacking gel and 10% (w/v) separating gel15. SDS-PAGE was performed using Mini-Protean Tetra System (Bio Rad, USA) at 19 mA for stacking and 36 mA for separating gels. Samples were prepared by mixing with sample buffer (0.05 M Tris, pH 8.8, 2% SDS, 5% sucrose, and 0.02% bromophenol blue) at a 1:1 (v/v) ratio. Then, samples were heated at +95 ºC for 1 min before loading into the gel. The total amount of proteins was 20 µg per well. The gels were stained with 2.5% Coomassie brilliant blue R-250 in 10% (v/v) ethanol, 10% (v/v) acetic acid, and 15% (v/v) isopropanol. Furthermore, the molecular weights of proteins were calculated using a protein calibration mixture (Bio Rad, USA).
Zymography was carried out according to the following method16. The separating gel solution (12%) was polymerized in the presence of gelatin (1 mg per mL), and samples were not heated to save enzymatic activity. After electrophoresis, the gels were soaked in 2.5% Triton Х-100 solution with gentle shaking (30 min at +25°C) to remove SDS and renature proteins. Next, the gels were washed with distilled water for 10 min to remove Triton X-100 and then incubated in 50 mM Tris-HCl pH 7.5 at +37 °C for 12 h. The clear areas on the gel indicate the presence of active enzymes. The TotalLab 2.04 program was used to analyze the resultant electropherograms. The represented electropherogram and zymogram are typical for a series of repeated experiments (at least three in each series).
Statistical analysis
Statistical analysis was performed with Statistica 8.0 software, and figures were plotted using OriginLab 9.1 version software. The data of biochemical estimations were reported as mean ± SEM for each group (n = 10). In addition, the Kolmogorov-Smirnov test was used to verify the normal distribution of results. Statistical analyses were performed using a one-way analysis of variance (ANOVA). Differences were considered to be statistically significant when p < 0.05.
Blood level of homocysteine in the rats of experimental groups
|
Experimental groups |
Homocysteine level, µmol·L-1 |
|---|---|
|
Control_young |
5.45±0.44 |
|
HC_young |
17.58±1.77* |
|
Control_adult |
5.65±0.91 |
|
HC_adult |
18.15±0.76 ** |
|
Control_old |
7.35±0.39 |
|
HC_old |
21.82±2.15*** |
Level of LMWS in the rats of experimental groups
|
Experimental groups |
Blood plasma LMWS, rel. units·(mL of blood) -1 |
Thyroid gland LMWS, rel. units·(g of tissue)-1 | ||
|---|---|---|---|---|
|
Registered at 254 nm |
Registered at 280 nm |
Registered at 254 nm |
Registered at 280 nm | |
|
Control_young |
0.32 ± 0.03 |
0.22 ± 0.05 |
2.87 ± 0.11 |
3.51 ± 0.16 |
|
HC_young |
0.62 ± 0.06 |
0.72 ± 0.07 |
7.63 ± 0.34* |
8.56 ± 0.40* |
|
Control_adult |
0.55 ± 0.09 |
0.29 ± 0.03 |
3.81 ± 0.17 |
2.23 ± 0.11 |
|
HC_adult |
1.45 ± 0.07 |
0.97 ± 0.10 |
7.64 ± 0.35** |
8.92 ± 0.43** |
|
Control_old |
0.70 ± 0.09 |
0.31 ± 0.04 |
4.71 ± 0.23 |
1.71 ± 0.075 |
|
HC_old |
2.75 ± 0.10 |
1.01 ± 0.05 |
9.52 ± 0.46*** |
9.36 ± 0.45*** |
Level of cytokines in the thyroid gland of rats of experimental groups
|
Experimental groups |
Pro-inflammatory cytokines |
Anti-inflammatory cytokines | |||||
|
|
TNFα |
INFγ |
IL-1b |
IL-6 |
IL-8 |
IL-4 |
IL-10 |
|
rel. units·(mg of protein)-1 | |||||||
|
Control_young |
29.5 ± 2.3 |
49.7 ± 2.3 |
43.5 ± 4.2 |
50.7 ± 3.8 |
33.0 ± 6.2 |
24.5 ± 3.4 |
55.0 ± 3.4 |
|
HC_young |
76.5 ± 3.6* |
63.2 ± 3.4* |
87.2 ± 4.4* |
91.5 ± 2.9* |
92.0 ± 4.2* |
78.2 ± 5.7* |
23.5 ± 1.7* |
|
Control_adult |
43.2 ± 1.2 |
56.7 ± 3.7 |
44.2 ± 1.9 |
46.5 ± 4.7 |
36.7 ± 4.9 |
26.5 ± 1.8 |
90 ± 4.9 |
|
HC_adult |
87.7 ± 4.5** |
66.7 ± 1.9** |
107.5 ± 3.9** |
101.2 ± 5.6** |
93.5 ± 3.7** |
77.5 ± 5.2 |
42.7 ± 2.7** |
|
Control_old |
158.3 ± 2.5 |
60.0 ± 3.5 |
46.3 ± 2.7 |
43.7 ± 2.7 |
39.1 ± 4.2 |
27.6 ± 2.3 |
74.9 ± 5.1 |
|
HC_old |
115.6 ± 5.3*** |
120.0 ± 6.7*** |
110.2 ± 5.6*** |
109.8 ± 4.5*** |
107.1 ± 5.5*** |
65.5 ± 4.7*** |
31.8 ± 2.7*** |
Results
Levels of LMWSin the blood and thyroid gland of HC rats
To confirm the state of HC, the plasma level of homocysteine was evaluated. This parameter increased 3.22, 3.21, and 2.96 times in the rats of the HC_young, HC_adult, and HC_old groups compared with the corresponding controls (
It was found that HC causes an increase in the level of LMWS in the blood plasma (
It should be noted that the physiological level of LMWS increased with age and was 2.87 ± 0.11 rel. units∙(g of tissue)in the rats of group Control_young, 3.81 ± 0.17 rel. units∙(g of tissue)in the rats of group Control_adult, and 4.71 ± 0.23 rel. units∙(g of tissue)in group Control_old rats. According to the obtained data, the level of LMWS registered at a wavelength of 280 nm changed more significantly. This value increased 2.4-fold in rats of the HC_young group, fourfold in the HC_adult group, and fivefold in the rats of group HC_old.
Cytokine profile in the thyroid gland of HC rats
A significant increase in the contents of all pro-inflammatory cytokines in the thyroid gland of the rats with HC was observed compared with the corresponding controls (

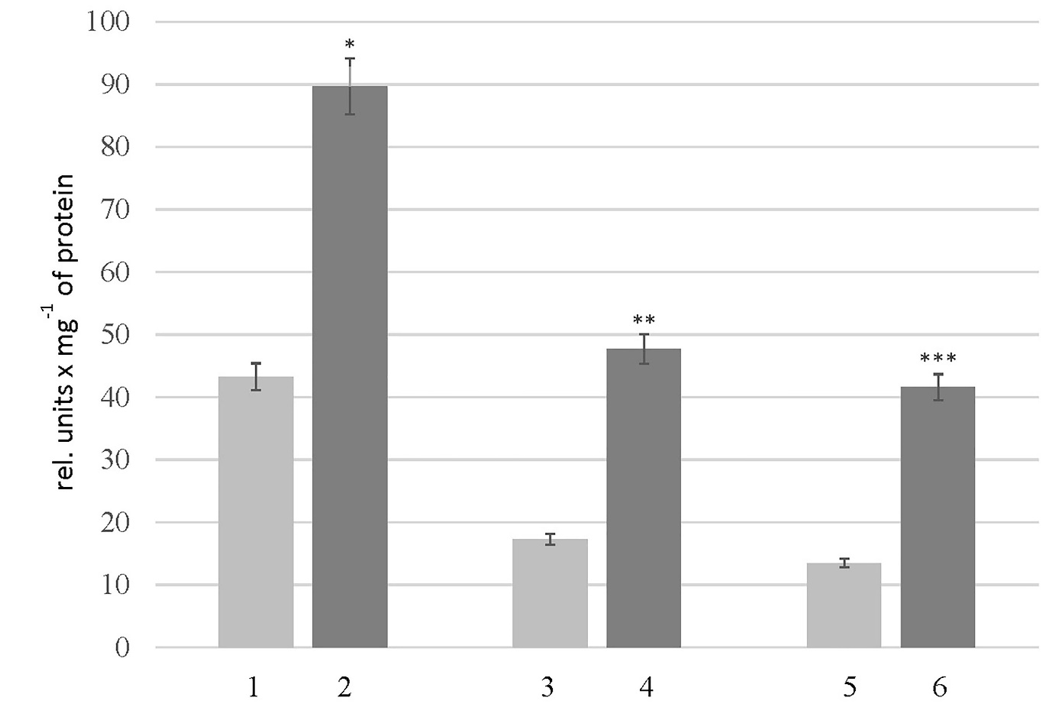
Level of peptides in the thyroid gland of rats of experimental groups. 1 – “Control_young”; 2 – “HC_young”; 3 – “Control_adult”; 4 – “HC_adult”; 5 – “Control_old”; 6 – “HC_old”. The data is presented asmean ± SEM (n = 10); *p < 0.05 significantly different from the group “Control_young”; **p < 0.05 significantly different from the group “Control_adult”; *** p < 0.05 significantly different from the group “Control_old”

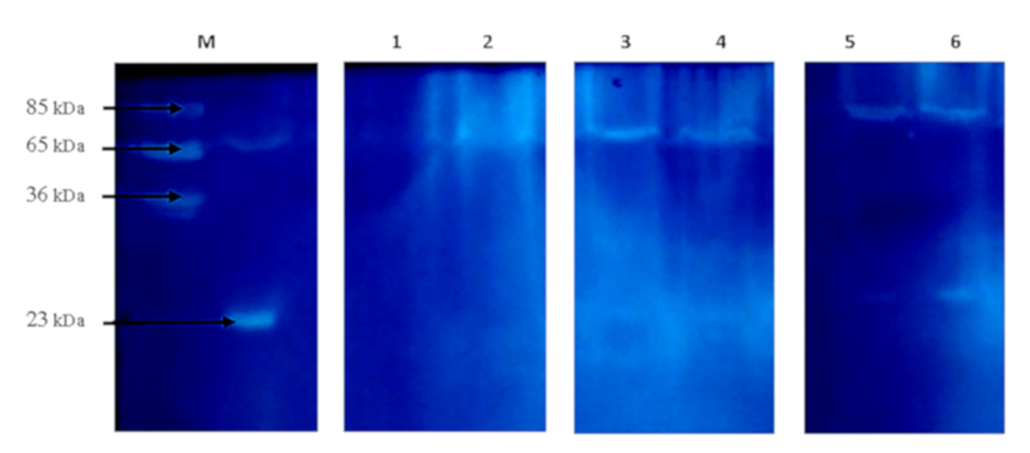
Electropherogram of the thyroid gland samples of rats of experimental groups. M – molecular weights markers (кDа); 1 – “Control_young”; 2 – “HC_young”; 3 – “Control_adult”; 4 – “HC_adult”; 5 – “Control_old”; 6 – “HC_old”
Protein composition (%) in the thyroid gland of rats of experimental groups
|
Protein fraction, kDa |
Experimental groups | |||||
|
Control_young |
HC_young |
Control_adult |
HC_adult |
Control_old |
HC_old | |
|
> 150 |
6.92 ± 0.34 |
11.41 ± 0.56* |
6.50 ± 0.23 |
- |
4.71 ± 0.21 |
- |
|
150 - 100 |
0.81 ± 0.03 |
2.38 ± 0.11* |
6.42 ± 0.23 |
6.00 ± 0.27** |
8.68 ± 0.41 |
5.11 ± 2.47*** |
|
100 - 70 |
1.72 ± 0.08 |
2.10 ± 0.10* |
1.61 ± 0.06 |
1.45 ± 0.06** |
2.45 ± 0.11 |
1.20 ± 0.03*** |
|
70 - 50 |
29.53 ± 1.47 |
13.54 ± 0.67* |
15.84 ± 0.75 |
8.63 ± 0.41** |
14.61 ± 0.71 |
5.17 ± 2.50*** |
|
50 - 30 |
1.81 ± 0.08 |
4.79 ± 0.23* |
5.35 ± 0.36 |
7.21 ± 0.33** |
4.88 ± 0.22 |
3.54 ± 0.16*** |
|
˂ 30 |
59.36 ± 2.71 |
65.5 ± 3.11* |
64.1 ± 3.11 |
76.55 ± 3.78** |
64.74 ± 3.21 |
84.96 ± 4.15*** |
Zymogram of the thyroid gland samples of rats of experimental groups. M –molecular weights markers (кDа); 1 – “Control_young”; 2 – “HC_young”; 3 – “Control_adult”; 4 – “HC_adult”; 5 – “Control_old”; 6 – “HC_old”. Assay was performed using gelatin (1 mg per mL) polymerized in 12% separating gel.
Level of MMP-2 in the thyroid glands of rats of experimental groups. 1 – “Control_young”; 2 – “HC_young”; 3 – “Control_adult”; 4 – “HC_adult”; 5 – “Control_old”; 6 – “HC_old”. The data is presented as mean ± SEM (n = 10);*p < 0.05 significantly different from the group “Control_young”; **p < 0.05 significantly different from the group “Control_adult”; ***p < 0.05 significantly different from the group “Control_old”
Level of peptides and protein composition in the thyroid gland of HC rats
A significant decrease was observed in the level of peptides in the rats of the HC_young group (2.4 times) compared to the thyroid glands of the control animals. The opposite changes were found in the rats of the HC_adult and HC_old groups, where peptides levels increased 1.7 and 1.2 times, respectively (Figure 1). Findings further revealed some changes in the protein composition, which were more pronounced in aged rats with HC (Figure 2,
Overall proteolytic activity in the thyroid gland of HC rats
The presence of active enzymes was analyzed using zymography. Following the principle of this method, the appearance of clear areas on the dark background of gel indicates the presence of active enzymes; the more intense the areas, the more active the enzymes are. In addition, this method can be used to estimate the apparent molecular weight of active enzymes. As shown in Figure 3, the samples of thyroid glands of the control rats and rats with HC have the same number of clear areas, but the intensity of these areas differed as there are two bands at the enzyme-electropherogram. One is localized in the region corresponding to 65-80 kDa proteins, and the other is in the region of 25 kDa proteins. Since zymography was performed using gelatin as a substrate protein, clear bands in the region of 65-80 kDa may be associated with the action of MMP-2 and/or MMP-9, the molecular weight of which is 65 kDa and 85 kDa. Therefore, the level of MMP-2 in the thyroid glands of rats with HC was analyzed. As depicted in Figure 4, HC was accompanied by a 2-fold increase in MMP-2 level in the rats of group HC_young. This parameter in the rats of groups HC_adult and HC_old was 2.7 and 3.1 times higher than the corresponding controls.
Discussion
According to literature data and our previous studies, the pathogenesis of many diseases is accompanied by the formation and accumulation of LMWS in tissues, which is considered a nonspecific manifestation of the disease. The fraction of LMWS is heterogeneous by nature and consists of substances with a molecular weight up to 5,000 Da18, 19. It should be noted that most low-molecular-weight substancesare typical for normal metabolism and can be found in minimal concentrations under physiological conditions. However, a situation can change dramatically in the case of pathologies associated with oxidative imbalance, inflammation, or/and enhancement of proteolysis. These conditions can potentiate an increase in catabolism resulting in the accumulation of low-molecular-weight breakdown products. Increasing the concentrations of metabolites above physiological values is potentially dangerous because it can affect the biochemical reactions and provoke the disruption of homeostasis within specific organs or even influence overall homeostasis.
To evaluate whether HC leads to the accumulation of LMWS, the level of these substances in the blood and thyroid gland of the rats was assessed. In order to provide a more comprehensive estimation, the measurement was performed at wavelengths of 254 and 280 nm. LMWS measured at 254 nm is considered a critical parameter reflecting the presence of non-aromatic sulfur-containing molecules, purine bases (adenine, guanine), and free nucleotides. In comparison, a measurement of LMWS at 280 nm was applied to detect aromatic chromophores (phenols, tyrosine, tryptophan, and phenylalanine). It has been established that HC causes an increase in LMWS in the blood, which is an unfavorable marker of this disease. The accumulation of LMWS in the blood may further indicate a decrease in the ability of the body’s detoxification systems to neutralize and eliminate the excess of these substances. A marked increase in the level of LMWS in the thyroid gland of HC rats was also revealed. This can be explained by an intensification of catabolic processes in the thyroid gland of rats with HC simultaneously with the disorders of the mechanisms responsible for the elimination of LMWS. It should be emphasized that more pronounced changes in adult and old animals of control groups may reflect the age-related changes in the compensatory metabolic potential in the thyroid gland.
Recent studies have indicated that serum homocysteine level is correlated to inflammatory markers, namely pro-inflammatory cytokines. Moreover, Shu Meng et al. concluded that homocysteine possesses pro-inflammatory activity by inducing inflammatory transcriptional signal pathways20. Given the above, and because numerous thyroid disorders are accompanied by persistent inflammation in the organ, the level of cytokines in the thyroid gland in HC rats was studied. Findings revealed that the cytokine profile in the thyroid gland undergoes changes under HC conditions as the level of pro-inflammatory cytokines increases, which may suggest the state of inflammation.
It should be emphasized that cytokines are not the only markers of inflammation but are also actively involved in modulating this state, both positively and negatively. For example, they can increase the inflammatory response by stimulating immune cells in the thyroid gland, which leads to the maintenance of inflammation and may provoke disturbances in the functional activity of the thyroid gland. In addition, the possible adverse effect of cytokines on the thyroid gland may be due to their ability to modulate the immune response, affecting the balance between the induction of autonomy and autoimmunity21. Clinical reports show that specific cytokines are involved in the progression of autoimmune thyroid disease by mediating direct damage to thyroid follicular cells. It has been shown that IL-10 acts as an inflammation inhibitor and is also involved in the induction/maintenance of tolerance and prevention of autoimmunity22, 23. Therefore, a decrease in the cytokine level and an increase in the levels of pro-inflammatory cytokines can be considered part of the pathogenic mechanism of thyroid gland damage in HC.
Results also showed a significant increase in the level of peptides in the thyroid gland of adult and old animals. According to the modern concept24, the sum of peptides in a tissue represents the peptide pool, vital in maintaining local homeostasis. Due to a broad spectrum of activities, peptides of the pool are actively involved in regulating the nervous, endocrine, immune, and cardiovascular systems25. However, significant and/or long-term changes in the physiological composition and level of certain peptides in the pool are considered factors that can affect biochemical processes. Thus, peptides that accumulate in tissues due to increased degradation of proteins against the background of impaired excretion can block cell receptors non-specifically, bind to active and/or regulatory sites of enzymes, and affect gene expression. An appearance of low molecular weight proteins, a decrease in the part of high molecular weight proteins, and peptide accumulation are indirect evidence of protein homeostasis disorders in the thyroid gland of rats with HC. However, the mechanisms responsible for increased protein degradation in HC are not entirely established. It can be suggested that a high concentration of homocysteine causes N-homocysteinylation of proteins, which is a signal for their removal from the cell to avoid toxicity. In addition, oxidative modification of proteins under HC-induced oxidative stress is another mechanism by which proteins are targeted for degradation.
Activation of proteases due to an increase in their level or violation of the coordinated pathways of regulation of their activity may be another explanation for the redistribution of proteins of different molecular weights. In this experiment, it was established that HC is accompanied by proteolysis enhancement in the thyroid gland of rats with HC. Furthermore, an increase in the level of MMP-2 was revealed. It is well-known that gelatinases are specific for basement membrane proteins such as type IV collagen, fibronectin, and laminin. Moreover, recent studies have shown that MMP-2 can degrade extracellular matrix collagen26. Given the multiple biological functions of ECM, the overexpression of MMP-2 can potentially lead to the degradation of extracellular matrix proteins and the impairment of cellular hemostasis. Nowadays, data confirm the involvement of MMPs in the progression of inflammation. In particular, it has been established that the cleavage of some cytokines by MMP-2 generates their active forms27. Therefore, the increase of MMP-2 levels in the thyroid gland of HC rats can be one of the causes of an increase in the level of active cytokines.
Conclusions
The association between disorders in the functional activity of the thyroid gland and the concentration of homocysteine has been established. However, to our knowledge, there are no studies dedicated to the investigation of the mechanisms to explain these disorders. Given the significant role of thyroid hormones in maintaining the body’s metabolic status, it is essential to understand how HC can affect the thyroid gland. The data obtained in this study indicate the catabolic effect of HC, which manifests itself in the accumulation of LMWS in the blood and thyroid gland and as increased levels of peptides. This may partly be the result of protease activation, as evidenced by increased total proteolytic activity and MMP-2 levels in the thyroid gland of HC rats. A change in the protein profile, namely, the accumulation of proteins with a molecular weight of less than 30 kDa with a simultaneous decrease in the level of high molecular weight proteins, is an additional confirmation of uncontrolled proteolysis. The state of inflammation, evidenced by the increased pro-inflammatory cytokines, was also revealed. All these changes can affect the functional state of the thyroid gland in HC. A significant limitation of the present work is that functional tests have not been performed to assess the functional activity of the thyroid gland in rats with HC. Further studies are needed to assess the impact of HC on the functional state of the thyroid gland and to understand how the identified changes can be associated with the development of thyroid disorders.
Abbreviations
HC: hyperhomocysteinemia, LMWS: low-molecular-weight substances, MMP: matrix metalloproteinases
Acknowledgments
None.
Author’s contributions
Drafting the article and data interpretation (Raksha Nataliia), carried out the experiments (Halenova Tetiana, Dzevulska Iryna, Kaminsky Rostyslav, Yanchyshyn Andrii), manuscript review (Zaichko Nataliia, Samborska Inga), experimental design (Maievskyi Oleksandr, Savchuk Olexiy), supervised the study (Kovalchuk Оleksandr). All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All experiments on animals were performed in the compliance with international principles of the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes (Strasbourg, 1986). The study was approved by the Ethical Committee of Taras Shevchenko National University of Kyiv (protocol №3 approved 25.11.2020).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

