
The outcomes of mechanical thrombectomy in patients with acute stroke with and without intravenous injection of tissue plasminogen activator
- Department of Neurology, School of Medicine, Sina (Farshchian) Educational and Medical Center, Hamadan University of Medical Sciences, Hamadan, Iran
- Research Center for Health Sciences, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract
Background: Stroke is a major health problem and leading cause of mortality worldwide. This study aimed to evaluate the consequences of intravascular interventions in patients with acute ischemic stroke with and without intravenous tissue plasminogen activator (tPA) injection.
Methods: This prospective cohort study was conducted on 120 patients with acute ischemic stroke. Patients were divided into three groups based on the type of disease treatment: angiography, tPA, and combined therapy (angiography + tPA). Patients in the tPA group were treated with a standard dose of intravenous alteplase (0.9 mg/kg). The severity of acute ischemic stroke was calculated on days 0, 14, and 90 after the interventions using the National Institutes of Health Stroke Scale (NIHSS) and Modified Rankin Scale (mRS) questionnaires.
Results: All three groups were homogeneous regarding age, gender, location, job status, education, and body mass index (BMI) distribution. Coronary disease was the most common risk factor for stroke in 38.33% of patients. There was no significant difference in mean NIHSS and mRS scores between the groups on days 0, 14, and 90 after intervention (p > 0.05). Mean NIHSS and mRS scores significantly decreased from the first time point (day 0), 10 days until 90 days after treatment in all three groups (p < 0.001); however, there was no significant difference in treatment effect between the three groups at these time points (p > 0.05).
Conclusion: Treatment with angiography and tPA alone or with combined therapy significantly decreased the severity of acute ischemic stroke. A significant decrease was observed in the mean NIHSS and mRS scores in all therapy groups, indicating that these treatments were effective in mitigating acute ischemic stroke and improving functional independence in these patients. The eligibility criteria by which patients with acute ischemic stroke are selected for each specific therapy are essential to achieving better clinical outcomes.
Introduction
Stroke is a major global health problem and is one of the top four leading causes of death in most countries1, 2. It is the most common type of neurological disease causing long-term adult disability worldwide, accounting for at least half of all neurological disorders in public hospitals3. Despite recent advances in stroke treatment, the disease remains a cause of morbidity and mortality and represents a heavy economic burden on society4. The prevalence of stroke in some countries is high; about 150 per 100,000 Iranian people suffer from stroke each year5. Furthermore, the mean age of stroke onset in Iran is lower than in modern societies. Stroke is classified into hemorrhagic and ischemic stroke; approximately 80% of strokes are ischemic6. Cardioembolic type, atrial fibrillation, atherosclerosis, hyperlipidemia, lacunar type, hypertension, dissections, vasculitis, COVID-19, and specific genetic disorders are important risk factors for strokes7, 8, 9, 10.
Prior to 1995, stroke treatment was based on the prevention of stroke recurrence and rehabilitation therapies; however, a revolution occurred in the treatment of acute stroke following a study published by the National Institute of Neurological Disorders and Stroke11. Patients within 3 hours of the onset of ischemic stroke were treated with intravenous tissue plasminogen activator (tPA) at a dose of 9.9 mg/kg body weight. Patients treated with tPA were at least 30% more likely to have minimal or no disability at 3 months on the assessment scales compared with the placebo group11. In further studies, the exclusion criteria for treatment were decreased and the time was increased to 4.5 hours. Although the number of patients eligible for treatment increased, the success rate of alteplase therapy remained low. Scientists found that involvement of large arteries inside the brain, such as the midbrain, did not respond or responded only slightly to tPA therapy. Therefore, intra-arterial therapies were introduced to address this problem. At first, the side effects of tPA therapy were significant due to improper selection of patients and the lack of appropriate intra-arterial devices and increased bleeding. Fortunately, with the introduction of new thrombectomy devices, such as stents and catheters that could reach the area near the intracerebral clot, intra-arterial therapy became one of the most effective treatments for acute stroke12. Previously, patients presenting within 6 hours of the onset of symptoms underwent thrombectomy; however, the time for a thrombectomy or intra-arterial was gradually increased, and a recent study showed that this time could be extended to 24 hours13. Therefore, the criteria by which patients are selected for each specific therapy is essential to achieving better clinical outcomes. To support this notion, a recent study demonstrated that patients with acute ischemic stroke and a history of taking antiplatelet drugs exhibited poor outcomes following administration of a standard dose of tPA3, indicating that intravenous tPA therapy should be applied with caution.
There are limited centers in Iran that are equipped to perform intra-arterial treatment. Sina Hospital, located in Hamadan (a city in Western Iran), is a well-known hospital in Iran that is equipped to perform intra-arterial therapy. Because intravascular interventions in ischemic stroke patients have not been evaluated properly and adequately in Iran, the present study aimed to investigate, for the first time, the consequences of intravascular interventions in Iranian patients with acute ischemic stroke with and without intravenous tPA injection. Moreover, this study includes a report about the usage of tPA in thrombectomy in acute stroke.
Methods
In this prospective cohort study, we recruited all patients who were admitted to Farshchian (Sina) Hospital (Hamadan, Iran) from the start of 2020 to the end of 2021 with acute ischemic stroke confirmed by clinical signs and brain computed tomography (CT). The study was approved by the Ethics Committee of Hamadan University of Medical Sciences (Ethics code: IR.UMSHA.REC.1398.1050). At the beginning of the study, a checklist was provided through which baseline characteristics of the patients, such as age, gender, body mass index (BMI), location, job, and education, were recorded.
The inclusion criteria were: patients with (i) ischemic acute stroke, (ii) age between 18 and 85 years, (iii) National Institutes of Health Stroke Scale (NIHSS) score between 6 and 22, and (iv) maximum start of intravenous therapy 4.5 hours after the stroke. Patients with a history of renal failure, liver disease, cancer, active infectious diseases, trauma or surgery within the last month, brain herniation, dementia, femoral artery failure, platelet < 50,000, high INR, cerebral hemorrhage, or systemic symptoms were excluded from the study.
Patients were divided into three groups based on the type of disease treatment: angiography, tPA, and combined therapy (angiography + tPA). Patients in the tPA therapy group were treated with a standard dose of intravenous alteplase (0.9 mg/kg). Brain CT was performed for every patient before receiving intravenous tPA. Patients were followed up with CT or magnetic resonance imaging (MRI) 24 hours after tPA infusion (Figure 1). The eligibility criteria for patients in each group are summarized in
The eligibility criteria for patients in each group
|
tPA therapy group |
Angiography therapy group |
tPA + angiography therapy group |
|---|---|---|
|
Diagnosis of ischemic stroke |
No active bleeding or impaired coagulation tests |
Diagnosis of ischemic stroke |
|
Onset of symptoms within the last 4.5 hours |
Refer after 4.5 hours from the onset of symptoms |
Onset of symptoms within the last 4.5 hours |
|
Normal CT scan or lesion in imaging less than 3.1 MCA |
Contraindication to receiving tpa |
Normal CT scan or lesion in imaging less than 3.1 MCA |
|
Willingness for receiving tpa |
Willingness for angiography |
No response to receiving tpa |
|
No active bleeding or impaired coagulation tests |
Suitable artery puncture |
Willingness for angiography |
|
No active bleeding or impaired coagulation tests |
Suitable artery puncture |

A flowchart of patients’ selection and follow-up.
The severity of acute ischemic stroke was calculated on days 0, 14, and 90 after intervention using the NIHSS and Modified Rankin Scale (mRS) questionnaires. The NIHSS questionnaire comprised 11 different items, including level of consciousness (LOC), LOC questions, LOC commands, best gaze, visual, facial palsy, motor arm, motor leg, limb ataxia, sensory, best language, dysarthria, extinction, and inattention14. The score for each ability was a number between 0 and 4, 0 being normal functioning and 4 being completely impaired. The patient’s NIHSS score was calculated by adding the score for each element of the scale; 42 was the highest possible score. In the NIHSS, a higher score indicates more impairment in the stroke patient: patients with an NIHSS score < 5 have mild ischemic acute stroke, while patients with NIHSS scores 6 – 24 and ≥ 25 have moderate and severe ischemic acute stroke, respectively. The mRS is commonly used as a functional outcome of global disability for stroke studies, coded from 0 (no symptoms at all) to 5 (severe disability) and 6 (death)15. Good and poor outcomes were defined as mRS ≤2 and mRS >2, respectively. Patients with an mRS score from 0 to 2 have a high ability to carry out their daily activities, while patients with an mRS score from 3 to 5 are unable to carry out their daily activities.
Statistical analysis
All quantitative data are presented as mean ± standard deviation (SD). Crosstabs and Chi-square tests were used to compare the percentage or frequency of parameters between two groups. Comparison of the mean of parametric data, such as the NIHSS and mRS scores, between two groups, was performed using Student’s independent samples t-test. One-way ANOVA and Tukey post hoc test were applied to compare mean values of parametric data between the three groups. Moreover, changes in the NIHSS and mRS scores over time between the three groups were analyzed using the repeated-measures ANOVA test. In this study, p< 0.05 was considered statistically significant. SPSS software version 19 (IBM Corp.) was used for data analysis.
Results
From April 2020 through September 2021, a total of 120 eligible patients were enrolled in our study. A total of 36 patients undergoing angiography were assigned to intra-arterial intervention, 42 patients were assigned to intravenous injection of tPA, and 42 patients were assigned to receive combination therapy with intravenous alteplase and intra-arterial intervention (combination therapy group).
The baseline characteristics of the patients in the three groups are presented in
For patients who received tPA, the time to tPA therapy was less than 10 minutes for 4 patients, and 32 patients underwent tPA therapy 30 minutes after admission to our hospital. The tPA dose for all patients was 0.9 mg/kg. In 52 of 78 patients who underwent angiography, the angiography was performed more than 6 hours after stroke; only for 18 patients was the angiography performed less than 4 hours after stroke. Coronary disease was the most common risk factor for stroke in 38.33% of patients, followed by hypertension, hyperlipidemia, smoking, and diabetes in 31.67%, 23.33%, 21.6%, and 11.67%, respectively. The distribution of the associated risk factors for acute stroke according to the treatment type is presented in Figure 2.
The baseline characteristics of the patients
|
Variable |
Treatment group |
P value | |||
|
Angiography therapy n (%) |
tPA therapy n (%) |
Combination-therapy n (%) | |||
|
Number |
36 |
42 |
42 |
- | |
|
Mean age (year) |
56.33±17.1 |
64.95±14.74 |
56±13.15 |
0.01 | |
|
Gender |
16 (44.44) |
24 (57.14) |
24 (57.14) |
12 (57.14) |
0.44 |
|
20 (55.56) |
18 (42.86) |
18 (42.86) |
9 (42.86) | ||
|
Location |
30 (83.33) |
34 (80.95) |
40 (38.46) |
20 (38.46) |
0.12 |
|
6 (16.67) |
8 (19.05) |
2 (4.76) |
1 (4.76) | ||
|
Job |
10 (27.78) |
14 (33.33) |
12 (28.57) |
6 (28.57) |
0.58 |
|
4 (11.11) |
4 (9.52) |
6 (14.29) |
3 (14.29) | ||
|
20 (55.56) |
16 (38.10) |
18 (42.86) |
9 (42.86) | ||
|
2 (5.56) |
8 (19.05) |
6 (14.29) |
3 (14.29) | ||
|
Education |
16 (44.44) |
20 (55.56) |
10 (23.81) |
5 (23.81) |
0.03 |
|
8 (22.22) |
8 (22.22) |
14 (33.33) |
7 (33.33) | ||
|
8 (22.22) |
6 (16.67) |
6 (14.29) |
3 (14.29) | ||
|
4 (11.11) |
2 (5.56) |
12 (28.57) |
6 (28.57) | ||
|
BMI (kg/m2) |
20 (55.56) |
28 (66.67) |
24 (57.14) |
12 (57.14) |
0.18 |
|
14 (38.89) |
8 (19.05) |
10 (23.81) |
5 (23.81) | ||
|
2 (5.56) |
6 (14.29) |
8 (19.05) |
4 (19.05) | ||
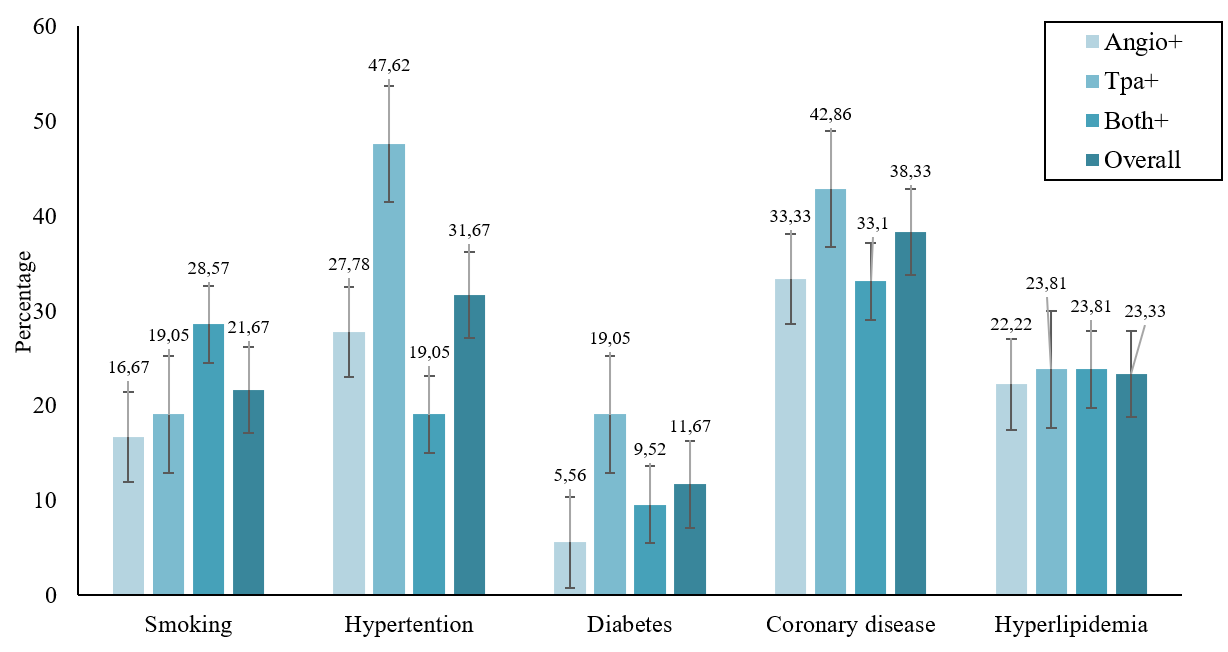
Distribution of risk factors for acute stroke patients according to the study groups.
Comparison of NIHSS and mRS scores between three groups of patients 14 and 90 days after intervention
|
Variable |
Treatment group |
Day 0 |
Day 14 |
Day 90 |
Time effect |
Treatment effect |
|---|---|---|---|---|---|---|
|
NIHSS |
Angiography therapy |
20.55 ± 3.77 |
10.35 ± 8.28 |
5.56 ± 6.87 |
< 0.001 |
0.77 |
|
tPA therapy |
21.86 ± 3.73 |
12.29 ± 9.42 |
4.84 ± 6.48 | |||
|
Combination-therapy |
22.57 ± 2.97 |
10.62 ± 7.49 |
4.25 ± 3.79 | |||
|
p value |
0.21 |
0.04 |
0.54 |
- |
- | |
|
mRS |
Angiography therapy |
4.28 ± 1.003 |
2.89 ± 2.02 |
1.81 ± 1.80 |
< 0.001 |
0.31 |
|
tPA therapy |
4.19 ± 1.06 |
2.19 ± 1.67 |
1.5 ± 1.88 | |||
|
Combination-therapy |
4.48 ± 0.67 |
2.71 ± 1.50 |
1.71 ± 1.80 | |||
|
p value |
0.35 |
0.35 |
0.18 |
- |
- | |

The changes in NIHSS and mRS scores in the days of 0, 14 and 90 after intervention.
Comparison of the mean NIHSS and mRS scores between the three groups of patients on days 0, 14, and 90 after intervention is presented in Figure 2 and
Following the 90-day follow-up after intervention, we observed three cases of inguinal hematoma and one case of intracerebral hemorrhage in the angiography therapy group, and one case of intracerebral hemorrhage and one death in the tPA therapy group. Moreover, in the combination therapy group, we observed four cases of inguinal hematoma and two case of intracerebral hemorrhage.
Discussion
In this study, we compared the severity of acute stroke between patients receiving tPA, angiography, or combined tPA + angiography therapy at different periods. Our data revealed that coronary disease was the most common risk factor for acute stroke in 38.33% of patients, while hypertension was the second most common cause of acute stroke in these patients. Furthermore, we found that the severity of acute stroke in all groups receiving angiography, tPA, and combined therapies significantly decreased from baseline until days 14 and 90 after treatment. The mean NIHSS score significantly decreased from ~21 at baseline to ~10 on day 14 and eventually ~5 on day 90. Interestingly, the mean mRS score significantly decreased from ~4 at baseline to ~2.5 on day 14 and eventually ~1.5 on day 90. These data indicated that both angiography and tPA therapies were invaluable for mitigating acute stroke and improving functional independence in patients with acute ischemic stroke. More importantly, the criteria by which patients are selected for each specific therapy is essential to achieving better clinical outcomes. More recently, Khazaei . 3 investigated the effect of previous administration of antiplatelet drugs on adverse outcomes of tPA therapy in patients with acute ischemic stroke.Patients were examined during a period of 3 hours after stroke and received a standard dose of tPA (0.9 mg/kg). They found that patients with a history of antiplatelet drug use had an increased rate of intracranial hemorrhage. Furthermore, NIHSS and mRS scores were significantly higher in patients receiving antiplatelet drugs than the other group at discharge and on day 90 after treatment. The severity of the disease decreased with time in both groups. The mortality rate was 42.1% in patients who received antiplatelet drugs and 18.5% in the other group. They concluded that patients with acute ischemic stroke and a history of taking antiplatelet drugs had poor outcomes following administration of a standard dose of tPA3. Therefore, intravenous tPA therapy should be used with caution. This finding is in line with the findings of our study. We found that the criteria by which patients were selected for each specific therapy, either for tPA or combined tPA + angiography, was an important step to achieving better clinical outcomes. In another study, Krajíčková . 16 evaluated the safety and efficacy of mechanical thrombectomy with stent retrievers in patients with anterior circulation stroke. They found that mechanical thrombectomy was safe and effective in patients on oral anticoagulation. Pierot . 17 demonstrated that 60% of patients with acute stroke in the stentriever group exhibited a significant decrease in their NIHSS score 24 hours after intervention. Furthermore, 54% of patients in the stentriever group had an mRS score between 0 and 2 within 90 days of treatment. Moreover, 71.1% of patients achieved successful recanalization, with a cerebral infarction score of 2b (mTICI-2b). These findings are consistent with the results of our study. Similarly, in our study, more than 50% of patients exhibited an improvement in NIHSS and mRS scores 24 hours after treatment. In another study, Bracard . 18 investigated whether mechanical thrombectomy in addition to intravenous thrombolysis could improve clinical outcomes in patients with acute ischemic stroke. Their findings revealed that 42% of patients with intravenous thrombolysis alone and 53% of patients with intravenous thrombolysis plus mechanical thrombectomy achieved functional independence at 3 months. There was no significant difference in the mortality rate at 3 months or symptomatic intracranial hemorrhage at 24 hours between the two groups. Vasospasm (23%) and embolization (6%) were the most common adverse events reported in both groups. These data indicate that mechanical thrombectomy combined with standard intravenous thrombolysis improves functional independence in patients with acute cerebral ischemia without increasing mortality. Touma . 19 considered the benefits and risks of using stent retrievers in addition to tPA for the treatment of acute ischemic stroke. They reported that, compared with tPA alone, the use of stent retrievers combined with tPA was associated with significant improvement in functional independence 90 days after acute ischemic stroke; however, there was no significant difference between the two groups regarding mortality rate19. In contrast, in our study, we did not find a significant difference in the improvement of functional independence 90 days after acute ischemic stroke between the tPA, angiography, and combined tPA + angiography groups. Therefore, it appears that selecting patients based on specific inclusion criteria for each therapy is an important factor in achieving acceptable clinical outcomes in these patients. Goyal . 20 considered the effect of endovascular treatment in addition to standard care in patients with acute ischemic stroke. They found that the rate of functional independence was significantly increased in the intervention group (53.0%) compared with the control group (29.3%). The mortality rate in the intervention group (10.4%) was significantly lower than in the control group (19%). Furthermore, symptomatic intracerebral hemorrhage was reported in 3.6% of participants in the intervention group and 2.7% of participants in the control group20. Therefore, according to the reported data and findings of our study, mechanical thrombectomy with or without intravenous injection of tPA is the preferred technique for the management of acute ischemic stroke. However, appropriate selection of patients based on defined eligibility criteria is an essential step for each therapy. Nevertheless, another study with a larger sample size is needed to support our findings because our small sample size was considered a limitation of the study.
Conclusions
In summary, our findings indicated that coronary disease was the most common risk factor for acute stroke. The severity of acute ischemic stroke in patients receiving angiography, tPA, and combined therapy (tPA + angiography) significantly improved from baseline to the end of the study. A significant decrease was observed in the mean NIHSS and mRS scores in all therapy groups. These data indicate that treatment with angiography and tPA alone or in combination are effective in mitigating acute ischemic stroke and improving functional independence in these patients. Most importantly, the eligibility criteria by which patients with acute ischemic stroke are selected for each specific therapy is an essential step for achieving better clinical outcomes.
Abbreviations
LOC: level of consciousness, MRI: magnetic resonance imaging, mRS: Modified Rankin Scale, NIHSS: National Institutes of Health Stroke Scale, tPA: tissue plasminogen activator
Acknowledgments
Deputy of Research and Technology of Hamadan University of Medical Sciences approved our study (Research code: 9905213220). We would like gratefully acknowledge the medical staff of Sina Hospital.
Author’s contributions
MK, RMM and MGH developed the research idea and proposal of the study. SHM, MK, MGH and RMM participated in data gathering. SK analyzed the data. All authors contributed to writing the manuscript. All authors approved the final version of the manuscript.
Funding
This study was funded by the Deputy of Research and Technology from Hamadan University of Medical Sciences, Iran (Research code: 9905213220).
Availability of data and materials
The data that support the findings of the study are available from the corresponding author in SPSS form upon reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The ethics committees of Hamadan University of Medical Sciences was approved the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

