
CAPE Improves Vanin-1/AKT/miRNA-203 Signaling Pathways in DSS-induced Ulcerative Colitis
- Molecular Biology Department, Genetic Engineering and Biotechnology Research Institute (GEBRI), Sadat University, Egypt
- Biochemistry Department, Faculty of Applied Medical Sciences, October 6 University, Egypt
Abstract
Introduction: Ulcerative colitis (UC) and other inflammatory bowel diseases (IBDs) are common chronic, inflammatory gastrointestinal diseases. Due to their antioxidant, anti-inflammatory, and antibacterial properties, polyphenols are beneficial in the treatment of IBD. Caffeine acid phenethyl ester (CAPE) has been shown to have cytotoxic, antibacterial, antioxidant, and anti-inflammatory effects. This study focuses on the biochemical and molecular levels of the mode of action of CAPE in DSS-induced UC in rats.
Methods: Thirty male Wistar rats were distributed into five groups, with six rats in each group: group I was administered 3 mL of distilled water orally, group II was administered CAPE (10 mg/kg.b.w.) orally, group III was administered 5% DSS orally, group IV was administered 5% DSS and CAPE (10 mg/kg.b.w.) orally; and group V was administered 5% DSS and sulfasalazine (100 mg/kg b.w.) orally.
Results: Individually, oral treatment with CAPE or sulfasalazine significantly ameliorated body weight, DAI score, and colon length in DSS-induced colitis and raised blood PLT count, NO, NF-kß, and vitamin C levels. In addition, animals given CAPE had a considerable increase in colon GSH, GPx, CAT, and SOD levels compared with rats given DSS. Compared with the DSS control group, colon TBAR, IL-6, and INF-ɣ were lower in the CAPE-treated rats. Histopathological examination revealed that CAPE treatment caused tissue injury and improved vanin-1, AKT, and miRNA-203 genes in the distal colon and triggered apoptosis.
Conclusions: The gastroprotective impact of CAPE was more noticeable than sulfasalazine. CAPE treatment caused biochemical and histopathological improvements, indicating that CAPE may have antioxidant and anti-inflammatory effects in colitis; therefore, CAPE may be a potential therapeutic agent for the amelioration of IBD. This finding is promising for future therapies and research goals.
Introduction
Several factors, including genetics, microbiome, and environmental stressors, are the causes of ulcerative colitis (UC)1, 2, 3. In UC, the epithelial cell lining of the colon becomes inflamed4, 5, 6, 7. Dextran sulphate sodium (DSS) is a polysaccharide with varying molecular weight ranging from 5 to 1400 kDa8. Due to its toxicity to colonic epithelial cells, DSS promotes human UC-like diseases, resulting in depressed mucosal barrier function9. Weight loss, diarrhea, and occult blood in the stool are common observations in the DSS-treated rat model10. Many documents have demonstrated the pharmaceutical importance of phytochemicals in reducing UC symptoms, enhancing immune activity, and providing antioxidants that reduce inflammation in animal models11, 12, 13. However, the data indicate a need for further studies to elucidate the benefits and mechanisms of these compounds.
Caffeic acid phenethyl ester (CAPE) is a major active phenolic compound of some types of propolis14, 15. It has been shown to be protective against oxidative stress-mediated tissue damage16, 17, 18, 19. CAPE has been associated with a variety of and pharmacological effects20, 21, 22, 23, and there have been reports of its gastroprotective activity in animal models. In addition, its anticancer properties were observed in the skin of mice treated with bee propolis and exposed to 12-O-tetradecanoylphorbol-13-acetate24, 25.
Our findings indicate that the presence of vanin-1 in tissues of the epithelium influences the perception of stress26 by innate immune cells as an inhibitor of inflammatory processes and the treatment of colitis27. In addition, research indicates the upregulation of miRNA 203 and AKT gene expression in certain inflammatory tissues and organs28, 29, 30. As a measure of our interest research program in the treatment of inflammatory diseases31, 32, 33, 34, 35, 36. In the present study, we aimed to evaluate the therapeutic potential of CAPE in DSS-induced UC in rats.
Methods
Chemicals
All chemicals were purchased from Sigma Aldrich, Germany.
- Caffeic acid phenethyl ester (97%) powder: Formula [CHO] molecular weight (284.31), CAS. No. (104594-70-9).
- Dextran sulfate sodium (98%) powder: Formula [(CHNaOS)n], molecular weight (>500,000), CAS. No. (9011-18-1).
- Sulfasalazine (99%) powder: Formula (CHNOS), molecular weight (398.394), CAS. No. (599-79-1).
Animals
Male albino rats weighing 150 ± 10 g each were donated by the National Cancer Institute Animal House at Cairo University in Giza, Egypt. They were kept in plastic cages with stainless steel covers at a humidity level of 55 — 60% and a temperature of 22 °C in a light-controlled environment. The animals were maintained for 2 weeks to adapt and were provided regular feed and water at will.
Design of experiment
This experiment was designed to evaluate the gastroprotective effect of CAPE in DSS-induced UC. According to the guidelines of the Faculty of Applied Health Sciences Committee, the present study was design. The treatment grouping is described in
Description of treatment groups
|
Group NO. |
Groups |
Treatment description |
|---|---|---|
|
I |
Normal control |
Received 3 mL of distilled water, orally for 15 days. |
|
II |
CAPE (10 mg/kg.b.w.) |
Received 10 mg / kg bw. CAPE, orally, daily for 15 days |
|
III |
Positive control DSS, 5% in distilled water |
Received DSS, 5% orally, for 15 days |
|
IV |
DSS + CAPE |
Received DSS + CAPE, orally, for 15 days |
|
V |
DSS + Sulfasalazine (100 mg/kg b.w.) |
Received DSS + sulfasalazine (100 mg/kg b.w.), orally, for 15 days |
Calculation of disease activity index
In experimental colitis, the disease activity index (DAI) was calculated according to the method outlined by Bang and Lichtenberger38.
Sample collection
On day 16, 1 day after the last dose, blood samples were collected in tubes containing heparin from the retroorbital venous plexus of each animal. Heparinized blood samples were divided into two parts: the first part was used to estimate PLT count using a Sysmex KX-21N automated hematology analyzer (Sysmex Corp., Kobe, Japan)39, and the second part was centrifuged at 1000 g for 20 min. Separated plasma was used to estimate plasma levels of NF-κB using an ELISA kit (MyBioSource Inc., San Diego, CA, USA, 92195-3308), NO using the calorimetrically calibrated diagnostic kit, interleukin 6 (IL-6) using an ELISA kit (Abcam plc, USA), and vitamin C (Vit. C) using an ELISA kit (Novus kits, Novus Biologicals, LLC, California, USA).
At the end of the experiment, the colon of each animal was excised, washed with phosphate-buffer saline (PBS), gently stretched, and the distance between the colocecal junction and the distal end of the rectum was measured40. The distal sections of the colons were then separated; one piece was used for histopathological analysis, and the other piece was kept frozen at 80 °C until biochemical analysis of TBARS, GSH, SOD, and CAT41, 42, 43, 44 using colorimetric methods in the diagnostic kit. In addition, the IFN-γ content was determined using an ELISA kit (Abcam plc, USA).
Real-time PCR
Using the RNA-spinTM (QiaGen GmbH, Hilden, Germany), total RNA from colon tissues was extracted45. As instructed by the manufacturer, cDNA was used for qPCR using the SYBR Green PCR master mix (iNtRON Biotechnology, Korea). The reverse transcription kit was used to produce cDNA from 1 – 5 g total RNA (Applied Biosystems, Foster City, CA). The sequences of the genes evaluated (vanin-1, AKT, and miRNA-203) and the housekeeping primer used in RT-PCR, β-actin (Primer Design Ltd, USA), are shown in
The primer sequences
|
Gene |
Sequences | |
|---|---|---|
|
Vanin-1 |
forward |
5'-AACTGGATACCCTGTGATAACCC-'3 |
|
reverse |
5'- GTCTCCCATGTTCGCCACAA-'3 | |
|
AKT |
forward |
5'-CCCTGCTCCTAGTCCACCA–'3 |
|
reverse |
5'-TGTCTCTGTTTCAGTGGGCTC-'3 | |
|
miRNA-203 |
forward |
5'- GGGGTGAAATGTTTAGGAC-'3 |
|
reverse |
5'- CAGTGCGTGTCGTGGAGT-'3 | |
|
β-actin (housekeeping) |
forward |
5'-TGACTGACTACCTCATGAAGATCC-'3 |
|
reverse |
5'-TCTCCTTAATGTCACGCACGATT-'3 | |
Histological examination
A sample of colon tissues was collected and fixed in 10% neutral buffered formalin. It was then dehydrated in ascending graders of ethyl alcohol (50–100%), cleared in xylene and embedded in melted paraffin wax (MP 59), embedded in paraffin as blocks, and 5–6-micron-thickness sections were cut using a rotary microtome46. The paraffin sections were stained and exanimated using a light microscope (Olympus, Münster, Germany). A photomicrograph of the colon tissue was taken at 400 x magnification.
Statistical analysis
Data are presented as mean ± standard deviation (SD) for six measurements for both spectrophotometric measurements and ELISA. However, there are three separate determinations for PCR analysis of gene expression. All data were analyzed using SPSS version 20 software47. The one-way analysis of variance (ANOVA) test was used to assess the hypotheses. Statistical significance was defined as P < 0.05.
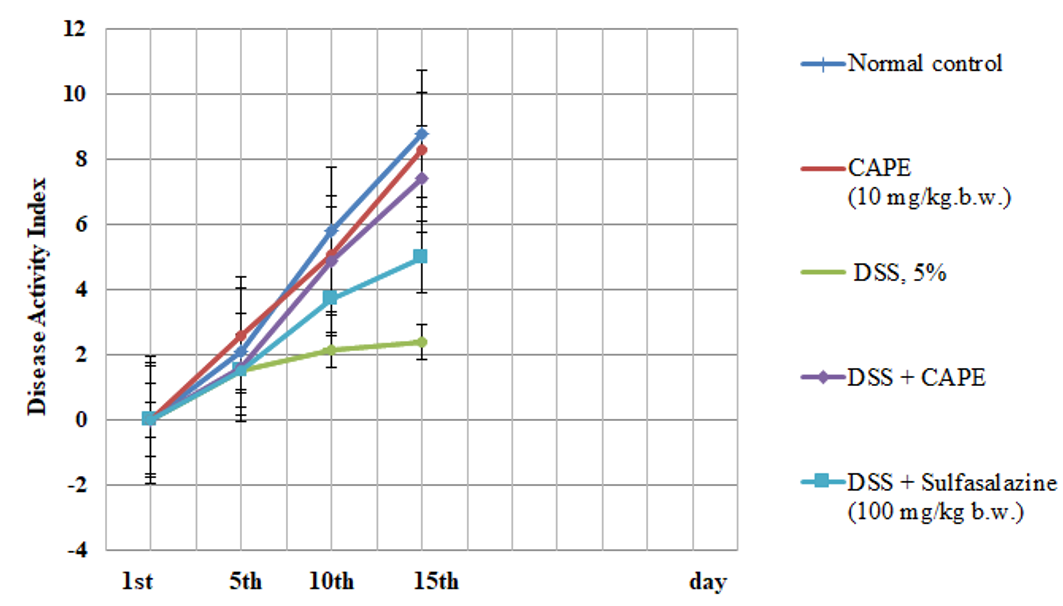
Effect of CAPE and sulfasalazine on disease activity index in DSS induced colitis groups of rats for 15 days. Data was expressed as mean ± SEM (n = 6). The obtained values were significantly different at P ≤ 0.05.
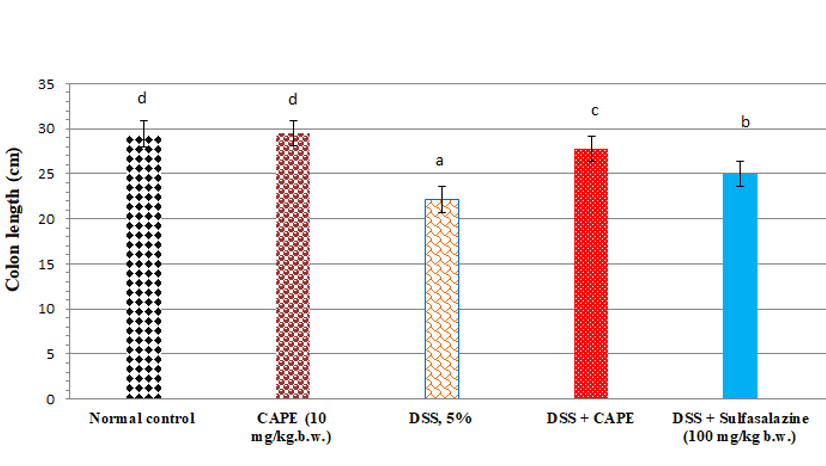
Effect of CAPE and sulfasalazine on colon length in DSS treated rats. Data was expressed as mean ± SEM (n = 6).

Effect of CAPE and sulfasalazine on colon Vanin-1, AKT and miRNA-203 gene expression in DSS-treated rats. Representative bar diagram of three independent experiments is presented. Data followed by the same letter are not significantly different at P ≤ 0.05.
Effect of CAPE and sulfasalazine on the body weight every 5 days of DSS treated rats
|
No. |
Groups |
Number of days/ Body weight of rats(g) | |||
|---|---|---|---|---|---|
|
1 |
5 |
10 |
15 | ||
|
(I) |
Normal control 3 mL of distilled water, orally |
160.42 ± 3.72bA |
163.72 ± 5.08aA |
169.79 ± 7.69 |
174.63 ± 3.24cC |
|
(II) |
CAPE (10 mg/kg.b.w.) |
162.29 ± 8.53A |
166.63 ± 8.23AB |
170.58 ± 10.54 C |
175.69 ± 8.06cD |
|
(III) |
Positive control DSS, 5% in distilled water |
158.15 ± 10.78 aA |
160.50 ± 9.29aA |
161.57 ± 10.73 a |
161.99 ± 4.95 aB |
|
(IV) |
DSS + CAPE |
160.43 ± 10.45 A |
163.01 ± 7.72aA |
168.33 ± 5.28ab |
172.37 ± 9.62 C |
|
(V) |
DSS + Sulfasalazine (100 mg/kg b.w.) |
159.73 ± 4.47aA |
162.07 ± 6.14aAB |
165.70 ± 8.00aBC |
167.73 ± 6.03aC |
Effect of CAPE and sulfasalazine on blood PLT count as well as plasma NO, NF-kβ and Vit.C in DSS-treated rats
|
Groups |
Treatment Description |
PLT (103/mL) |
NO (ng/mL) |
NF-kβ (ng/mL) |
Vit. C (µmol/L) |
|---|---|---|---|---|---|
|
I |
Normal control 3 mL of distilled water, orally |
261.27 ± 23.18 c |
10.79 ± 1.87 a |
0.81 ± 0.10 a |
83.13 ± 7.96 d |
|
II |
CAPE (10 mg/kg.b.w.) |
265.33 ± 18.62 c |
9.67 ± 0.54 a |
0.93 ± 0.16 a |
82.04 ± 5.46 d |
|
III |
Positive control DSS, 5% in distilled water |
209.28 ± 12.08 a |
36.13 ± 4.39 d |
3.51 ± 0.37 d |
59.76 ± 6.46 a |
|
IV |
DSS + CAPE |
265.00 ± 26.38 c |
14.52 ± 3.30 b |
1.36 ± 0.22 b |
77.13 ± 6.14 c |
|
V |
DSS + Sulfasalazine (100 mg/kg b.w.) |
217.58 ± 14.00 B |
21.94 ± 2.56 c |
2.40 ± 0.31 c |
65.98 ± 5.25 b |
Effect of CAPE and sulfasalazine on colon GSH, GPx, CAT and SOD in DSS-treated rats
|
Groups |
Treatment Description |
GSH (nmole/mg protein) |
GPx (U/mg protein) |
CAT (U/mg protein) |
SOD (U/mg protein) |
|---|---|---|---|---|---|
|
I |
Normal control 3 mL of distilled water, orally |
29.89 ± 2.79d |
25.97 ± 2.85d |
32.67 ± 2.75d |
97.44 ± 11.13 d |
|
II |
CAPE (10 mg/kg.b.w.) |
30.71 ± 3.21d |
22.39 ± 3.77cd |
35.48 ± 3.62d |
96.17 ± 9.49 d |
|
III |
Positive control DSS, 5% in distilled water |
13.64 ± 2.65a |
8.98 ± 0.84a |
13.82 ± 2.27a |
53.09 ± 6.99 a |
|
IV |
DSS + CAPE |
23.04 ± 3.20 c |
19.28 ± 3.21c |
27.50 ± 2.23 c |
79.25 ± 6.48 c |
|
V |
DSS + Sulfasalazine (100 mg/kg b.w.) |
17.45 ± 3.35b |
13.78 ± 2.65 |
21.60 ± 2.44b |
61.19 ± 5.82b |
Effect of CAPE and sulfasalazine on colon TBARs, IL-6 and INF-γ in DSS-treated rats
|
Groups |
Treatment Description |
TBARs (nmol/mg protein) |
IL-6 (pg/mg protein) |
INF-γ (pg/mg protein) |
|---|---|---|---|---|
|
I |
Normal control 3 mL of distilled water, orally |
70.34 ± 5.72 a |
25.71 ± 4.49 a |
0.41 ± 0.06 a |
|
II |
CAPE (10 mg/kg.b.w.) |
68.83 ± 5.81 a |
27.85 ± 2.58 a |
0.38 ± 0.05 a |
|
III |
Positive control DSS, 5% in distilled water |
142.71 ± 8.73 d |
46.59 ± 4.69 d |
1.46 ± 0.13 d |
|
IV |
DSS + CAPE |
93.06 ± 8.06 b |
33.08 ± 4.13 b |
0.64 ± 0.08 b |
|
V |
DSS + Sulfasalazine (100 mg/kg b.w.) |
114.65 ± 11.44 c |
40.17 ± 4.25 c |
1.14 ± 0.17 c |
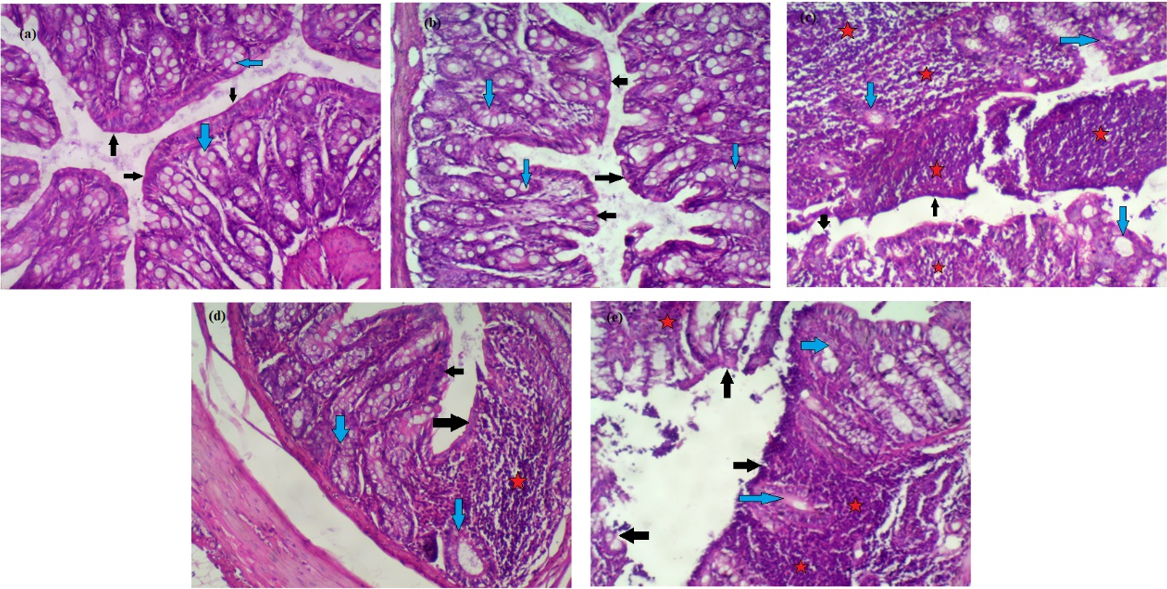
Effect of CAPE and sulfasalazine on histological changes of colon tissues of different groups. (a): Group I, (b): Group II: Was administrate CAPE (10 mg/kg.b.w.), (c): Group III: DSS (50%), (d): Group IV: Was administrate DSS (5%) + CAPE (10 mg/kg.b.w.), (e): Group V: Was administrate DSS (5%) + Sulfasalazine (100 mg/kg b.w.).
Results
Effect of CAPE and sulfasalazine on the body weight of DSS-treated rats
In contrast, administration of CAPE (10 mg/kg.b.w.) to DSS rats produced a significant increase in body weight by 60.40 % compared with the DSS-treated control group (P < 0.05). Administration of sulfasalazine (100 mg/kg b.w.) produced a non-significant increase in body weight in DSS-treated rats by 3.54 % when compared to the DSS-treated control group of rats.
Effect of CAPE and sulfasalazine on disease activity index in rats with DSS-induced colitis
The results in Figure 1 indicate a non-significant change in the DAI of CAPE-treated rats (group II) as compared to normal control rats (group I). However, the present data show a significant change in the DAI score after DSS treatment compared with the DAI of the control group. Treatment with CAPE (10 mg/kg.b.w.) and sulfasalazine produced significantly improved DAI scores in DSS-treated rats as compared with the group treated with DSS alone (P < 0.05).
Effect of CAPE and sulfasalazine on colon length in DSS-treated rats
The colon length of CAPE-treated normal rats (group II) was non-significantly changed when compared with the normal rats (Figure 2). Compared with the normal group of rats, treatment with DSS (5%) resulted in a significant reduction in colon length by 24.7 %. Furthermore, administration of CAPE to the DSS-treated group produced a significant increase in colon length by 25.38 % when compared with the DSS-treated control group (P < 0.05). However, the administration of sulfasalazine to DSS-treated rats produced a significant increase in colon length by 12.78% compared with the DSS-treated control group (P < 0.05).
Effect of CAPE and sulfasalazine on blood PLT count and plasma NO, NF-kβ, and vitamin C in DSS-treated rats.
Administration of CAPE to normal rats produced non-significant changes in plasma PLT, NO, NF-kβ, and Vit. C when compared with normal rats (
Compared with normal rats, DSS treatment (5%) resulted in a significant reduction in blood PLT count and plasma Vit. C levels by 19.89% and 28.11%, respectively, and a significant increase in plasma NO and NF-kβ by 234.84% and 333.30%, respectively (P < 0.05).
Additionally, compared with the DSS-treated control group, CAPE treatment resulted in significantly elevated PLT count and plasma Vit. C levels by 26.62% and 29.06%, respectively, and a significant decrease in plasma NO and NF-kβ by 59.81% and 61.25%, respectively (P < 0.05).
Sulfasalazine administration significantly increased blood PLT and plasma Vit. C levels by 3.96% and 10.4%, respectively, and produced a significant increase in plasma NO and NF-kβ by 39.27% and 31.62%, respectively, when compared with the DSS-treated control group (P < 0.05) (
Effect of CAPE and sulfasalazine on colonic GSH, GPx, CAT, and SOD in DSS-treated rats
Administration of CAPE to normal rats produced nonsignificant changes in colon GSH, GPx, CAT, and SOD when compared with normal rats. However, compared with normal rats, DSS (5%) treatment resulted in a significant reduction in colon GSH, GPx, CAT, and SOD of 54.36%, 55.42%, 57.69%, and 45.51%, respectively. Furthermore, administration of CAPE to rats treated with DSS produced a significant increase in colon GSH, GPx, CAT, and SOD by 68.91%, 114.64%, 98.9%, and 49.27%, respectively, when compared with rats treated with DSS alone (P < 0.05) (
Treatment of rats with sulfasalazine (100 mg/kg b.w.) produced a significant increase in colon GSH, GPx, CAT, and SOD by 27.93%, 53.45%, 56.29%, and 15.25%, respectively, compared with the DSS-treated control group (P < 0.05).
Effect of CAPE and sulfasalazine on colonic thiobarbituric acid-reactive substances (TBARs), interleukin 6 (IL-6), and interferon γ (INF-γ) in DSS-treated rats
Compared with the DSS-treated control group, CAPE administration in the X group resulted in a significant reduction in colonic TBAR, IL-6, and INF-γ by 35.22%, 28.99%, and 56.10%, respectively. Furthermore, administration of sulfasalazine (100 mg/kg b.w.) significantly decreased colonic TBARs, IL-6, and INF-γ by 19.66%, 13.77% and 21.91%, respectively, when compared with the DSS-treated control group (P < 0.05).
Effect of CAPE and sulfasalazine on colonic vanin-1, AKT, and miRNA-203 gene expression in DSS-treated rats
Figure 3 shows the individual effects of CAPE and sulfasalazine on the expression of the vnin-1, AKT and miRNA-203 genes in normal rats treated with DSS. Treatment of normal rats with CAPE (10 mg/kg.b.w.) produced a nonsignificant change in colon vanin-1, AKT, and miRNA-203 gene expression.
When compared with the normal control group, oral administration of DSS (5%) resulted in a significant increase in colon levels of vanin-1, AKT, and miRNA-203 gene expression by 416.66%, 511.65%, and 761.4%, respectively (P < 0.05).
However, compared with the DSS-treated control group, CAPE treatment resulted in a significant reduction in colonic expression of vanin-1, AKT, and miRNA-203 gene by 55.35%, 52.53%, and 72.41%, respectively. Furthermore, the administration of sulfasalazine to rats treated with DSS significantly decreased the expression of vanin-1, AKT, and miRNA-203 genes in the colon by 74.95%, 73.65%, and 55.17%, respectively, compared with the control group (P < 0.05).
Effect of CAPE and sulfasalazine on histological alterations in colon tissues
Figure 4(a-e) shows the individual effects of CAPE and sulfasalazine on colon histopathology of normal and DSS-treated rats.
In Figure 4a&b, histopathological examination of normal and CAPE-treated groups (I&II) revealed intact surface epithelium (black arrows) and regular glands with adequate mucin production (blue arrows). The lamina propria did not show inflammatory cell aggregates.
In Figure 4c, colon histopathological examination of DSS-treated rats revealed surface erosions and ulcerations (black arrows). Many glands were replaced by inflammation, with mucin depletion (blue arrows). Many lymphoid aggregates (stars) are observed.
Furthermore, oral administration of CAPE to rats treated with DSS produced an intact surface epithelium (black arrows); mucin depletion was partially corrected (blue arrows), and few focal lymphoid aggregates were still observed (stars) compared with rats treated with DSS.
However, histological examination of rats treated with sulfasalazine/DSS showed a partly eroded surface epithelium (black arrows) with glandular mucin depletion in many glands (blue arrows); large lymphoid aggregates were still seen (stars).
Discussion
UC is believed to be a TH2-mediated inflammatory disease, while Crohn's disease is thought to be a TH1-mediated inflammatory disease48. In animal models, the inflammatory imitate UC mediated by TH1 and TH 2 was elevated49.
Numerous studies have shown that polyphenols and flavonoids can be used therapeutically to prevent invasion and metastasis of colorectal cancer cells50, 51.
In that study, we suggested that CAPE inhibited the expression of inflammatory mediators and biomarkers of oxidative stress. A recent study reported a previously unknown mechanism in which CAPE could inhibit invasion and migration by modulating the MMP-2 and MMP-9 signaling pathways52.
Effect of CAPE and sulfasalazine on the body weight of DSS-treated rats
In UC animal models, the degree of inflammation is measured by determining the daily change in body weight of the animals during each experiment and measuring the length of the resected colon53, 54. Although weight loss alone is a poor predictor of well-being55, it is still accepted that weight loss >20% is a criterion for euthanasia and an indication that the experimental design may be too aggressive55. The three primary symptoms of IBD in DSS-induced rats with colitis were considerable weight loss, bloody diarrhea, and shortening of the colon56, 57. In addition, in rats treated with DSS, colon shortening may be related to thickening due to edema and infiltration of inflammatory cells into the lamina propria and submucosa58.
Effect of CAPE and sulfasalazine on colonDAI score and length in DSS treated rats
In our investigation, treatment of rats with DSS for 15 days caused acute colitis with an elevated DAI score and a decreased colon weight/length ratio. A substantial correlation between the DAI score and inflammation in DSS-induced acute and chronic colitis has been reported by Bullich .57 Following treatment with CAPE, colon length, DAI score, and body weight improved. Many researchers have reported the antioxidant, anti-inflammatory, anticancer, prebiotic, immunomodulatory, and gastroprotective properties of polyphenols and their metabolites59, 60. CAPE treatment led to a marked reduction in the inflammatory infiltrate in both the lamina propria and the submucosa and protected against changes in colon length in a dose-dependent manner. Espíndola .52 showed that CAPE inhibits the release of inflammatory cytokines from human hepatocellular carcinoma cells.
Effect of CAPE and sulfasalazine on blood PLT count and plasma NO, NF-kβ, and Vit. C in DSS-treated rats
In the present study, we observed a depletion of blood PLT levels in DSS-treated rats. Our results were in line with those reported by Zamora .61 and Honjo .,62 who showed that UC patients had the lowest levels of PLT and Vita. C due to the production of inflammatory mediators NO and NF-kβ63 and the depletion of endogenous antioxidant biomarkers (i.e., Vit. C), which reduced the binding of PLTs to monocytes through the membrane, favoring an inflammatory response in UC patients with onset flare. The PLT changes in DSS-treated rats were restored upon treatment with CAPE and sulfasalazine. CAPE exhibited potent antioxidant activity by restoring the levels of PLT, NO, NF-kβ, and Vit. C. Our results are in line with the results of Gupta 64 and Nakashima .65, who noticed that phenolics significantly reduced levels of inflammatory mediators, thereby suppressing their inflammatory response in UC. Our results suggest that CAPE treatment decreases the expression of NO and NF-kβ, increases the production of Vit. C, and ameliorates intestinal mucosal barrier dysfunction in UC.
Effect of CAPE and sulfasalazine on colon GSH, GPx, CAT, and SOD in DSS-treated rats
In the present study, we noticed depletion of colon GSH, GPx, CAT, and SOD in DSS-treated rats. Our results were in line with the results reported by Zieliska et al.66, who observed a significant decrease in GSH, GPx, CAT, and SOD levels in patients with IBD when compared with controls. Our results indicate that the administration of CAPE significantly improves macroscopic damage, colon length, increases the activity of GPx, CAT, and SOD, depresses TBARs and NO levels, and increases GSH levels in the colon tissues of experimental colitis. Many articles have shown that the administration of flavonoids and polyphenols modulates the levels of antioxidant biomarkers and inflammatory mediators67, 68.
Effect of CAPE and sulfasalazine on colonic TBARs, IL-6, and INF-γ in DSS-treated rats
Our study indicated the elevation of TBAR, IL-6, and INF-γ levels in the colon tissues of experimental colitis. Yan .69 reported elevation of TBAR, IL-6, and INF-γ levels in colon tissue. These markers increase the inflammatory response of IL-6, INF-γ, NF-κB, destructive enzymes, and TBARs that cause damage to colon tissue70. In our study, CAPE significantly ameliorated tissue damage, IL-6, INF-γ, NF-κB, and TBARs in rats treated with DSS. Furthermore, our results indicate that DSS upregulates colon vanin-1 gene expression in DSS-treated rats. Our results were confirmed by the results of Gensollen .71, who reported vanin-1 upregulation in the intestinal tract in DSS-induced colitis. Our study suggests that elevation of vanin-1 gene expression in DSS-treated rats is a direct target for NF-κB in the colon, and this could be related to susceptibility to UC.
Effect of CAPE and sulfasalazine on colon vanin-1, AKT, and miRNA-203 gene expression in DSS-treated rats
Colon Akt gene expression was significantly upregulated in DSS-treated in rats. Our results were in line with a study by Li .72, who reported upregulation of Akt in DSS-treated rats. Furthermore, the Akt signaling pathway was significantly inhibited by CAPE administration, leading to the recovery of intestinal microbiota diversity. Our results indicated the downregulation of colon Akt gene expression by CAPE administration due to its inhibitory activity against colon NF-κB production in DSS-treated rats. Additionally, our study revealed upregulation of miRNA-203 gene expression in DSS-treated rats. Tian .73 studied the effect of inflammation on miRNAs and found that the mucosa of UC patients that was infiltrated with inflammatory cells had elevated miRNA levels, while the levels were reduced to non-inflammatory levels in patients under remission. Further investigation revealed the role of miRNA in suppressing inflammatory mediator genes within colonic epithelial cells, all implicated in IBD74. However, miR-203 downregulation was observed in DSS-treated rats after oral CAPE administration. An association between vanin-1 and AKT suggests that overexpressed vanin-1 decreases the extent of AKT74 phosphorylation. Furthermore, miR-203 can activate the Akt signaling pathway through IL-8 in the regulation of radioresistance in nasopharyngeal carcinoma cells75. Moreover, the AKT signaling pathway has been shown to be effective in preventing ventilator-induced lung injury76. Our findings suggest regulation of miR-203 gene expression due to inhibition of IL-6, INF-γ, and NF-κB production and vanin-1 and Akt gene expression by CAPE administration.
Our results support and indicate downregulation of colon vanin-1 gene expression in DSS-treated rats after CAPE administration for 15 days due to inhibition of colon NF-κB levels.
Effect of CAPE and sulfasalazine on histological changes in colon tissues
In rats treated with DSS, histological analysis of colon tissue indicated altered mucosal architecture and inflammation. Our observations of microscopic changes were consistent with a large body of research on DSS-induced UC models in rats77. CAPE inhibits changes in colon architecture and length and significantly reduces inflammatory infiltration in both the lamina propria and submucosa.
CAPE treatment significantly decreased colon injury and contributed to the anti‑inflammatory and anti‑apoptotic effects. Furthermore, the anti‑inflammatory and anti‑apoptotic activities of CAPE and its effects on vanin-1, AKT, and miRNA-203 gene expression in IBD rats has not been previously documented, and this study may be the first of its kind.
Conclusions
The current study used biochemical and molecular analysis to show that IBD is associated with increased levels of oxidative stress and apoptosis. The data show that IL-6, INF-α, and NF-kβ are actively involved in gut mucosal inflammation in DSS-treated rats. Furthermore, we found significant improvement in miRNA-203, vanin-1, and Akt gene expression in DSS-treated rats. The potential benefits of CAPE on colon lipid peroxidation, inflammatory mediators, and antioxidant systems led researchers to hypothesize that it would be a viable choice for treating IBD.
Abbreviations
AKT: Protein kinase B, CAPE: caffeic acid phenethyl ester, CAT: catalase, DAI: disease activity index, DSS: dextran sulfate sodium, GPx: glutathione peroxidase, GSH: reduced glutathione, IL-6: interleukine-6, INF-γ: interferon-γ, IBD: inflammatory bowel disease, NO: nitric oxide, NF-kβ: nuclear factor kappa, PLT: platelet count, SOD: superoxide dismutase, TBARs: thiobarbituric acid reactive substances, Vit. C: vitamin C.
Acknowledgments
None.
Author’s contributions
All authors contributed significantly to this work, read, and approved the final manuscript.
Funding
None.
Availability of data and materials
Supporting data, including analytical (colorimetric ELISA-PCR) data, will be available to demonstrate the hepatoprotective activity of morin against paracetamol-induced liver toxicity.
Ethics approval and consent to participate
Data collection was ethically approved by the Research Ethics Committee of the Faculty of Applied Medical Sciences, October 6 University, Egypt (No. 20210614). lab studies formed the basis of this research, no human volunteers were used.
Consent for publication
The authors granted their permission for their personal information to be used in the article.
Competing interests
The authors declare that they have no competing interests.

