Double Vitrification-Warming Cycles Reduce Live Birth Rates in Single Euploid Blastocyst Transfers: A Retrospective Cohort Study
- Hai Phong University of Medicine and Pharmacy, Hai Phong City, Viet Nam
- 16A Ha Dong General Hospital, Ha Noi, Viet Nam
- Hanoi Medical University, Ha Noi, Viet Nam
Abstract
Objectives: To evaluate the impact of blastocysts biopsied once and vitrified twice on clinical outcomes.
Methods: This retrospective study analyzed 277 single euploid blastocyst transfer cycles conducted at the Center for Assisted Reproduction, 16A Ha Dong General Hospital, from March 2018 to January 2024. Cycles were stratified into two groups: Group BV (biopsied once, vitrified once; n = 207) and Group VBV (biopsied once, vitrified twice; n = 70). Pregnancy outcomes were compared between groups, and a binary logistic regression model identified variables associated with live birth rates.
Results: Group BV demonstrated superior outcomes compared to Group VBV across all metrics: implantation rate (55.6% vs. 37.1%, p < 0.001), clinical pregnancy rate (55.1% vs. 37.1%, p < 0.001), ongoing pregnancy rate (54.1% vs. 35.7%, p < 0.001), and live birth rate (53.6% vs. 35.7%, p = 0.01). The number of vitrification-warming cycles was the only factor significantly associated with reduced live birth rates (OR 1.95, 95% CI 1.01–3.78, p < 0.05).
Conclusions: An additional vitrification-warming cycle significantly reduces pregnancy success in single euploid blastocyst transfers. Patients undergoing PGT-A should be explicitly counseled about the potential decline in success rates if previously vitrified blastocysts undergo repeated warming cycles for diagnostic confirmation.
Introduction
Embryo cryopreservation has become a highly effective technique in assisted reproductive technology (ART). The application of single embryo transfer helps minimize the risk of multiple pregnancies and enhance cumulative pregnancy rates—both of which are significant benefits of embryo cryopreservation. Recent studies show that the outcomes of frozen embryo transfer (FET) cycles are at least comparable to those of fresh embryo transfer cycles1, 2. Furthermore, embryo cryopreservation plays a crucial role in preimplantation genetic testing for aneuploidy (PGT-A) by providing additional time to assess embryos for chromosomal abnormalities3. Combining trophectoderm biopsy with blastocyst cryopreservation in FET cycles is becoming increasingly common, but it raises several concerns that require further investigation. The primary advantage of blastocyst vitrification relies on the use of high concentrations of cryoprotective agents and rapid cooling rates, which prevent the formation of ice crystals within cells. This method has proven safe and effective. However, the effects of multiple vitrification-warming cycles on blastocysts are not fully understood4, 5, 6. Additionally, micromanipulation during trophectoderm biopsy, along with associated stressors, may adversely affect the implantation potential and survival of the blastocyst7, 8, 9. In many cases, cryopreserved blastocysts are warmed and biopsied for PGT-A to select for embryo sex, screen for newly discovered genetic disorders, or enhance outcomes after unsuccessful IVF cycles or miscarriages. These blastocysts typically undergo a single biopsy and two vitrification-warming cycles. Currently, there is a paucity of large-scale studies evaluating the effects of multiple vitrification-warming cycles on FET outcomes, and existing studies report conflicting results10, 11, 12, 13, 14, 15.
Therefore, this study aimed to compare the clinical outcomes of blastocysts undergoing a single biopsy followed by two vitrification-warming cycles with those undergoing a single biopsy and one vitrification-warming cycle in single euploid blastocyst transfer cycles.
MATERIALS AND METHODS
Materials
A total of 277 blastocysts were included in single euploid blastocyst transfer cycles in this study. This group consisted of 207 blastocysts biopsied and vitrified once and 70 blastocysts biopsied once and re-vitrified (vitrified twice). Data were collected at the Center for Assisted Reproduction, 16A Ha Dong General Hospital, from March 2018 to January 2024.
Methods
Research design & Ethical approval
Retrospective cross-sectional descriptive study.

Flow diagram of blastocyst preparation methods. Group BV (biopsied and vitrified once; n = 207): IVF (
IVF procedures
Oocyte retrieval, ICSI, and embryo culture: Under ultrasound guidance, cumulus-oocyte complexes (COCs) were retrieved and stored in G-MOPS™ PLUS medium (Vitrolife, Sweden). Following retrieval, COCs were incubated in G-IVF™ PLUS medium (Vitrolife, Sweden). After 3 ± 1 hours of incubation, cumulus cells were removed using GM501 Hyaluronidase medium (Gynemed, Germany). Mature oocytes at the metaphase II (MII) stage were selected for intracytoplasmic sperm injection (ICSI) under an inverted microscope. After ICSI, oocytes were cultured in 20–30 μL droplets of Global Total LP medium (CooperSurgical, USA) covered with LiteOil (CooperSurgical, USA) and maintained in an incubator at 5% O, 6% CO, and 37°C. Fertilization was assessed at 17 ± 1 hours post-ICSI, and cleavage-stage embryos were evaluated at 68 ± 1 hours post-ICSI. On day 3, assisted hatching was performed using a laser system with a 7.2-μm-diameter laser shot applied to the zona pellucida.
Blastocyst classification and biopsy
On days 5 and 6, blastocysts were graded using a simplified Gardner blastocyst grading system and categorized into four groups:
-
Good: Inner cell mass (ICM) and trophectoderm (TE) both graded AA.
-
Fair: AB or BA.
-
Medium: BB, AC, or CA.
-
Poor: BC, CB, or CC.
Trophectoderm biopsy was performed on hatching blastocysts, with 5–10 cells removed. Biopsied cells were washed three times in 1% PVP/PBS solution and transferred into PCR tubes containing 2.5 μL of washing solution. PGT-A was conducted using the MiSeq system (Illumina, USA).
Vitrification-warming and blastocyst transfer
Blastocysts were vitrified using the Cryotech Vitrification Kit 101 (Reprolife, Japan). Euploid blastocysts were warmed using the Cryotech Warming Solution Set Kit (Reprolife, Japan) prior to transfer. Transfers were performed when the endometrial thickness reached 7.0–13.5 mm.
Pregnancy diagnosis
β-hCG: Serum β-hCG concentration was measured in mIU/mL. A value <5 mIU/mL was considered non-pregnant (negative); ≥5 mIU/mL was considered pregnant (positive).
Biochemical pregnancy: Early pregnancy loss prior to ultrasound detection of a gestational sac.
Clinical pregnancy: Ultrasound-confirmed presence of a gestational sac (including ectopic pregnancy) 3 weeks post-positive β-hCG test.
Ongoing pregnancy: Ultrasound detection of fetal cardiac activity.
Live birth: Delivery of a live infant.
Statistical analysis
Analyses were performed using SPSS 22.0 (IBM, USA). Normally distributed continuous variables are reported as mean ± standard deviation; categorical variables as frequency (%). Independent-sample t-tests were used for continuous variables, and chi-square or Fisher’s exact tests for categorical variables. Binary logistic regression identified variables associated with live birth rates. Statistical significance was set at p < 0.05.
Results
In this study, we analyzed the single-embryo transfer outcomes of 277 euploid blastocysts, which were divided into two groups based on the number of vitrification-warming cycles: (1) blastocysts that underwent a single biopsy followed by one vitrification-warming cycle (group BV, n = 207) and (2) blastocysts that underwent a single biopsy followed by two vitrification-warming cycles (group VBV, n = 70), as shown in Figure 1.
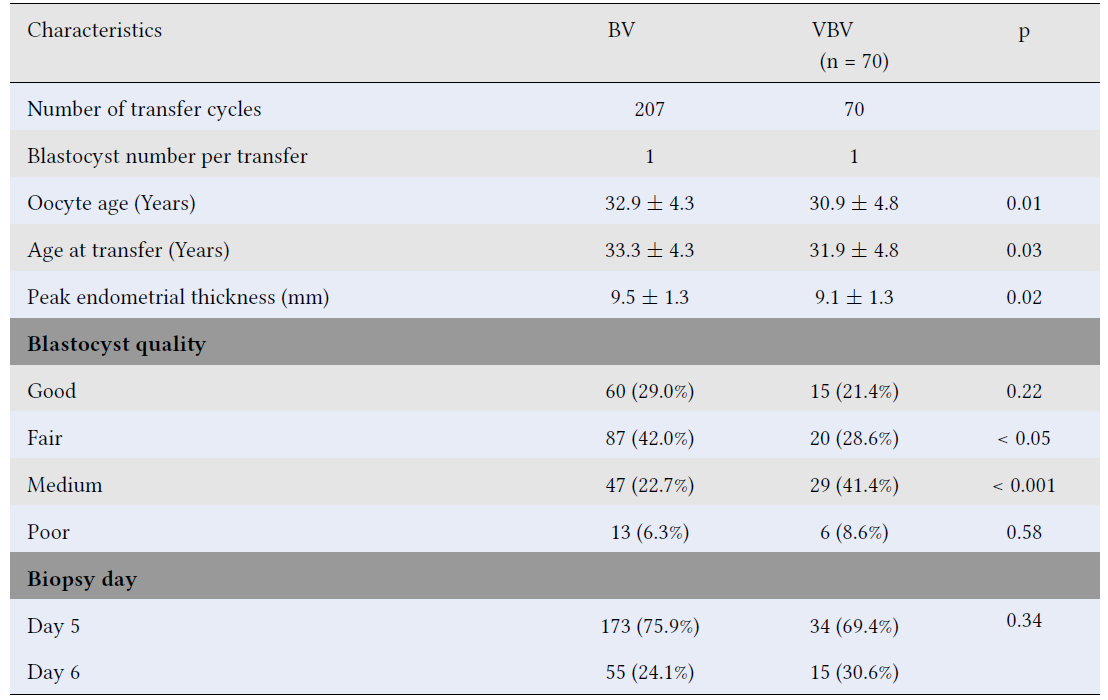
We found that oocyte age, age at transfer, and peak endometrial thickness in group BV were significantly higher than those in group VBV. Additionally, there was a higher proportion of good- and fair-quality blastocysts in the single vitrification group (group BV) compared to the double vitrification group (group VBV), whereas the proportions of medium- and poor-quality blastocysts were lower in group BV than in group VBV. However, statistically significant differences were only observed in the proportions of fair- and medium-quality blastocysts between the two groups. Furthermore, there was no significant difference in the distribution of Day 5 and Day 6 biopsied blastocysts between the groups. All patient and blastocyst characteristics are summarized in
Baseline characteristics of transfer cycles of euploid blastocysts vitrified once versus twice (group VBV) following a single biopsy
|
Characteristics |
BV |
VBV (n = 70) |
p |
|---|---|---|---|
|
Number of transfer cycles |
207 |
70 | |
|
Blastocyst number per transfer |
1 |
1 | |
|
Oocyte age (Years) |
32.9 ± 4.3 |
30.9 ± 4.8 |
0.01 |
|
Age at transfer (Years) |
33.3 ± 4.3 |
31.9 ± 4.8 |
0.03 |
|
Peak endometrial thickness (mm) |
9.5 ± 1.3 |
9.1 ± 1.3 |
0.02 |
|
Blastocyst quality | |||
|
Good |
60 (29.0%) |
15 (21.4%) |
0.22 |
|
Fair |
87 (42.0%) |
20 (28.6%) |
< 0.05 |
|
Medium |
47 (22.7%) |
29 (41.4%) |
< 0.001 |
|
Poor |
13 (6.3%) |
6 (8.6%) |
0.58 |
|
Biopsy day | |||
|
Day 5 |
173 (75.9%) |
34 (69.4%) |
0.34 |
|
Day 6 |
55 (24.1%) |
15 (30.6%) | |
The β-hCG positivity rate did not differ significantly between group BV and group VBV (127 [61.4%] vs. 37 [52.9%], p = 0.21). However, group BV demonstrated significantly higher implantation, clinical pregnancy, ongoing pregnancy, and live birth rates than group VBV: 115 (55.6%) . 26 (37.1%) for implantation (p < 0.001); 114 (55.1%). 26 (37.1%) for clinical pregnancy (p < 0.001); 112 (54.1%) . 25 (35.7%) for ongoing pregnancy (p < 0.001); and 111 (53.6%) . 25 (35.7%) for live births (p = 0.01). The biochemical pregnancy rate was approximately three times higher in group VBV (15.7%, n = 11) than in group BV (5.8%, n = 12) (
Pregnancy outcomes following single embryo transfer of euploid blastocysts subjected to single biopsy and either one or two vitrification-warming cycles
|
Pregnancy outcomes |
BV (n = 207) |
VBV (n = 70) |
p |
|---|---|---|---|
|
β-hCG positive |
127 (61.4%) |
37 (52.9%) |
0.21 |
|
Biochemical pregnancies |
12 (5.8%) |
11 (15.7%) |
< 0.001 |
|
Implantation |
115 (55.6%) |
26 (37.1%) |
< 0.001 |
|
Clinical pregnancies |
114 (55.1%) |
25 (37.1%) |
< 0.001 |
|
Ongoing pregnancies |
112 (54.1%) |
25 (35.7%) |
< 0.001 |
|
Live births |
111 (53.6%) |
25 (35.7%) |
0.01 |
Binary logistic regression analysis, adjusted for oocyte age, age at transfer, peak endometrial thickness, blastocyst quality, and biopsy day, revealed that exposure to two vitrification-warming cycles (vs. one) significantly reduced the likelihood of live birth (OR 0.51, 95% CI 0.27–0.99, p < 0.05). None of the other variables were significantly associated with live birth (p ≥ 0.05) (
Binary logistic regression analysis identifying variables associated with the likelihood of live birth
|
Variables |
OR |
95% CI |
p |
|---|---|---|---|
|
Oocyte age |
0.93 |
0.75 - 1.16 |
0.52 |
|
Age at transfer |
1.10 |
0.89 - 1.36 |
0.38 |
|
Peak endometrial thickness |
1.24 |
1.00 - 1.52 |
0.05 |
|
Blastocyst quality | |||
|
Good |
3.01 |
0.90 - 10.10 |
0.07 |
|
Fair |
1.45 |
0.44 - 4.81 |
0.55 |
|
Medium |
2.15 |
0.64 - 7.22 |
0.21 |
|
Poor |
1.00 |
0.09 | |
|
Biopsy day | |||
|
Day 5 |
1.65 |
0.82 - 3.30 |
0.16 |
|
Day 6 |
1.00 | ||
|
Number of vitrification-warming cycles | |||
|
Vitrified-warmed once |
1.95 |
1.01 - 3.77 |
< 0.05 |
|
Vitrified-warmed twice |
1.00 | ||
Discussion
PGT-A is a commonly used procedure in IVF to screen embryos for chromosomal abnormalities. It is typically recommended for patients with advanced maternal age, known chromosomal abnormalities, a heightened risk of chromosomal disorders in offspring, recurrent miscarriages, or repeated unsuccessful IVF cycles3, 7. Many vitrified blastocysts are thawed for trophectoderm biopsy to assess chromosomal status in patients meeting PGT-A criteria. Rather than undergoing additional ovarian stimulation cycles to obtain embryos of higher genetic quality, couples may opt for genetic testing on existing blastocysts to mitigate potential risks. Consequently, euploid blastocysts subjected to a single biopsy and two vitrification-warming cycles are occasionally used in frozen embryo transfer (FET) cycles. Although limited evidence exists on the success rates of these embryos, prior studies have explored their outcomes10, 11, 12, 13, 14, 15. This study aims to evaluate the clinical efficacy of transferring once-biopsied, twice-vitrified blastocysts.
When comparing outcomes between blastocysts undergoing one versus two vitrification-warming cycles (both biopsied once), pregnancy rates, implantation rates, and live birth rates were significantly lower in the twice-vitrified group. The live birth rate declined from 53.6% to 35.7%, highlighting the clinical relevance of this reduction for patient counseling and the potential advantages of pursuing a new stimulation cycle over reusing existing blastocysts (
Contrastingly, Huang . (2021) reported comparable pregnancy outcomes between twice-vitrified and once-vitrified blastocysts, advocating for the use of re-vitrified embryos to minimize waste6. Notably, their study excluded biopsied embryos and utilized Kitazato commercial media, potentially explaining discrepancies with our findings. Other studies, including Taylor (2014) and Theodorou (2022), found no significant differences in live birth rates between once- and twice-vitrified biopsied blastocysts10, 11, 14. Thus, the impact of repeated vitrification on embryo developmental potential remains debated, warranting further investigation.
Notably, oocyte age, age at transfer, endometrial thickness, and blastocyst quality differed between groups BV and VBV. After adjusting for confounders via logistic regression, only the number of vitrification-warming cycles significantly correlated with live birth rates (
Study limitations include its retrospective, single-center design, which may limit generalizability due to variability in laboratory protocols and culture media. The analysis also included a limited number of variables, potentially overlooking additional predictors of live birth. Furthermore, neonatal and perinatal outcomes were not evaluated. Prospective multicenter studies are needed to validate these findings and elucidate biological mechanisms affecting blastocyst competence.
Conclusions
Our findings demonstrate that blastocysts undergoing an additional vitrification-warming cycle and a single biopsy have reduced success rates compared to those undergoing a single biopsy and vitrification cycle. To reduce risks associated with additional ovarian stimulation and avoid discarding embryos, thawing vitrified blastocysts of unknown genetic status for biopsy in PGT-A candidates may be advisable. However, clinicians should clearly convey the reduced success rates associated with twice-vitrified blastocysts during counseling.
Abbreviations
Assisted Reproductive Technology (ART), Beta Human Chorionic Gonadotropin (β-hCG), Biopsied once and vitrified once (BV), Confidence Interval (CI), Cumulus-Oocyte Complexes (COCs), Frozen Embryo Transfer (FET), Inner Cell Mass (ICM), Intracytoplasmic Sperm Injection (ICSI), In Vitro Fertilization (IVF), Metaphase II (MII), Odds Ratio (OR), Polymerase Chain Reaction (PCR), Preimplantation Genetic Testing for Aneuploidy (PGT-A), Polyvinylpyrrolidone (PVP), Phosphate-Buffered Saline (PBS), Statistical Package for the Social Sciences (SPSS), Trophectoderm (TE), and Vitrified, warmed for biopsy, and re-vitrified (VBV).
Acknowledgments
We would like to express our sincere gratitude to the Board of Directors of the Fertility Center at 16A Ha Dong General Hospital, as well as Hai Phong University of Medicine and Pharmacy, for providing the necessary support and resources that enabled us to complete this article.
Author’s contributions
Thinh Ngo Van, Dung Le Thi Thuy and Luan Nguyen Thanh accquired the data. Thinh Ngo Van, Thuy Tran Thi, Dung Le Thi Thuy and Phuc Nguyen Hong analyzed the data and wrote the manuscript. Trang Nguyen Ha and Thinh Ngo Van revised the manuscript for important intellectual content and edited the manuscript. Tao Nguyen Dinh and Linh Pham Van critically revised and provided final approval of the version to be published. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
None.
Ethics approval and consent to participate
This retrospective study was approved by the Ethics Committee of 16A Ha Dong general Hospital. Informed consent was waived due to the use of de-identified patient records.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

