Impact of Antioxidant Supplementation on In Vitro Growth of Porcine Oocytes Derived from Early Antral Follicles
- Cellular Reprogramming Laboratory, School of Biotechnology, International University, Ho Chi Minh City, Viet Nam
- Vietnam National University, Ho Chi Minh City, Viet Nam
- University of Health Sciences, Ho Chi Minh City, Viet Nam
Abstract
Introduction: Oxidative stress (OS), a key factor impairing oocyte developmental competence, poses a major challenge in in vitro growth (IVG) systems. While antioxidant supplementation can mitigate OS-driven deficits, combining agents with complementary mechanisms—such as melatonin (Mela) and astaxanthin (Asta)—may offer synergistic benefits. This study evaluates the effects of Mela and Asta, individually and in combination, on oocyte quality, gene expression, and epigenetic markers during IVG.
Methods: Growing oocytes from early antral follicles (EAFs; 1–1.5 mm) were cultured for 4 days in a 3D agarose system. Initial experiments tested Mela (1, 10, 100 µM/mL) to assess survival rates and expression of BMP15, CAT, SOD1, and GPX4 via qPCR. Subsequently, the optimal Mela concentration was combined with Asta (0.05, 0.1, 0.25 µM/mL) to determine synergistic effects. Histone H3 methylation (Me-H3-K4) and global transcriptional activity (nascent mRNA accumulation) were analyzed to evaluate chromatin remodeling and transcriptional dynamics.
Results: Mela supplementation significantly improved oocyte survival compared to controls, with 1 µM/mL Mela yielding optimal results. Combining 1 µM/mL Mela with 0.05 µM/mL Asta further enhanced survival rates and upregulated antioxidant genes (CAT, SOD1, GPX4) and BMP15. This combination also elevated Me-H3-K4 levels and nascent mRNA accumulation, indicating enhanced transcriptional activity and chromatin remodeling. These changes correlated with reduced ROS impact and improved oocyte quality post-IVG.
Conclusion: Co-supplementation with Mela (1 µM/mL) and Asta (0.05 µM/mL) synergistically mitigates OS during IVG by boosting antioxidant gene expression, enhancing epigenetic modifications (Me-H3-K4), and promoting global transcription. This strategy significantly improves oocyte developmental competence, offering a promising approach to optimize IVG systems.
Introduction
growth (IVG) is a methodological procedure that facilitates the development of oocytes from early-stage follicles by establishing a supportive environment with proper timing and essential nutrient supplementation. This method may have the potential to produce a large number of meiotically competent oocytes following maturation (IVM), thereby enhancing the implications for successful assisted reproductive technologies. Especially, ovarian tissue cryopreservation (OTC) offers fertility preservation and restoration of endocrine function for female cancer patients prior to undergoing gonadotoxic treatments, although it remains an experimental approach. Reimplantation of OTC is currently the only option to use preserved tissue. However, this procedure often carries the potential risk of re-inducing malignancy. Early antral follicles (EAFs) serve as an intermediate stage between pre-antral and antral follicles, offering a promising source for obtaining mature oocytes because they are abundant and require a shorter culture duration compared with other pre-antral follicles (PAFs). However, challenges persist in culturing EAFs, and the efficiency of embryo production from EAF-derived oocytes remains substantially lower than that achieved with oocytes from small antral follicles (SAFs) and fully grown oocytes from large antral follicles (LAFs) in multiple mammalian species1, 2, 3, 4, 5. This disparity underscores the need for further research and development in IVG of growing oocytes from EAFs, and various modifications have been suggested to overcome this problem6, 7.
Based on changes and additions to the substrate to support the culture of growing oocytes, we have successfully used an agarose gel 3D matrix for the culture of preantral follicles, which has improved follicle growth and helped form fluid-filled cavities called antrum formation, marking the transition from preantral to antral follicles8. One of the main factors affecting reduced oocyte quality during culture is the production of reactive oxygen species (ROS), which cause DNA damage, protein misfolding, enzyme inactivation, cell apoptosis, and meiotic abnormalities8, 9. Supplementation of antioxidants is essential to reduce the harmful effects of ROS. We have successfully improved oocyte quality at the pre-IVM stage by supplementing the culture medium with ascorbic acid5.
Melatonin (N-acetyl-5-methoxy tryptamine; Mela) is produced by the pineal gland and plays an important role in regulating the circadian rhythm in mammals10, 11. This hormone is a powerful antioxidant, scavenging and neutralizing free radicals such as hydroxyl radicals, ROS, and reactive nitrogen species via its aromatic indole ring. As an antioxidant, it directly regulates the activity of several antioxidant enzymes, oxidative metabolism, and the electron transport chain12. The addition of Mela has improved blastocyst quality; however, the quality of blastocysts derived from EAFs remains lower than those from LAFs. Based on the positive effects of Mela on oocytes during the IVM stage, we supplemented it during the IVG stage. Mela at 100 µM has been shown to significantly improve oocyte quality after the IVM stage13. After further testing with Mela at 1 µM, 10 µM, and 100 µM during the IVG stage, we observed an improved blastocyst rate compared to the non-supplemented group; nevertheless, the quality and overall blastocyst rate remained significantly lower than in the control LAFs group14. Therefore, we aim to conduct this study to gain deeper insights into the effects of Mela during IVG culture.
Astaxanthin (3,3′-dihydroxy-β, β′-carotene-4,4′-dione; Asta), a xanthophyll carotenoid found in various microorganisms and marine organisms15, has numerous biological functions, including blocking lipid peroxidation, anti-inflammatory activity, and scavenging ROS and hydroxyl radicals16, 17. Asta, which is a nonenzymatic antioxidant, neutralizes free radicals18. Its antioxidant capacity is more powerful than other antioxidants, such as vitamins C and E or other carotenoids19. Supplementation with Asta is beneficial for the maturation of porcine and bovine oocytes, helping to prevent heat shock20, 21. OCGCs cultured with 0.5 mg/L (≈ 0.83 µM) Asta displayed improved quality compared to the non-treated group22. Asta also protects granulosa cells from oxidative stress by upregulating phase II enzymes through NRF2 activation23. Nevertheless, astaxanthin reduces granulosa cell growth and antrum formation24. Therefore, the effects of Asta on oocytes after the IVG stage remain ambiguous. Consequently, concentrations of 0.05, 0.1, and 0.25 µM were selected for this study. Specific gene expressions during this stage need to be evaluated. It has been suggested that the simultaneous and exclusive presence of Mela and vitamin C antioxidants in the IVF medium reduces ROS, thus enhancing embryo development up to the blastocyst stage25. Therefore, combining Mela and Asta antioxidants with different activities may have great potential to improve culture systems of growing oocytes from EAFs.
Porcine-growing oocytes isolated from EAFs (1–1.5 mm) predominantly exhibit uncondensed and dispersed chromatin in the germinal vesicle (GV, the oocyte nucleus), defined as filamentous/stringy chromatin (FC/SC) morphology26. At this stage, oocytes remain transcriptionally active and functionally connected with the surrounding cumulus cells through gap junctions. However, these oocytes cannot spontaneously resume meiosis or support embryo development. With the transition to LAFs (4–6 mm), the porcine oocyte undergoes significant changes associated with the completion of the growth phase, such as the shift from FC/SC to higher degrees of chromatin compaction, namely the GV stage26, 27. We reported that histone H3 acetylation/methylation occurs in chromatin throughout the nucleoplasm of growing oocytes. In fully grown porcine oocytes, chromatin transitions from a decondensed state to a heterochromatin ring around the nucleolus. This acetylation/methylation is crucial for transcriptional activation and increases during oocyte growth. In addition, histone H3 methylation at lysine 4 (Me-H3-K4) is regulated by specific nuclear factors during folliculogenesis26. Thus, assessing histone acetylation/methylation levels and chromatin structure can effectively predict the meiotic capacity of -grown oocytes.
Additionally, both Asta and Mela exert specific effects on gene expression. Mela indirectly reduces oxidative stress by stimulating the expression of many endogenous antioxidative enzymes (., superoxide dismutase 1 [SOD1], glutathione peroxidase [GSH]) and has an inhibitory effect on pro-oxidative enzymes28. Indeed, studies on the effect of Mela in IVG show an increase in the size of oocytes derived from the preantral follicle in mice29, sheep30, and secondary oocytes of goats31. During the growth stage of oocytes, essential antioxidant genes such as , Catalase (), and Glutathione Peroxidase 4 () are expressed to protect oocytes from oxidative stress32. This study also analyzed the expression of Bone Morphogenetic Protein 15 (), which is critical for folliculogenesis and oogenesis support factors33. Moreover, the accumulation of mRNA during oocyte growth plays a pivotal role in embryo quality at the preimplantation stage: mRNA synthesis increases during oocyte growth and is maintained for mature oocytes after fertilization34. Besides that, we reported that paternal chromatin undergoes extensive remodeling following sperm entry into the oocyte, with maternally derived RNAs rapidly degraded after fertilization, before zygotic gene activation35. Accordingly, protein synthesis relies predominantly on maternal mRNA that accumulates in the oocyte during the phase preceding zygotic gene activation. Hence, it is crucial to evaluate the expression of specific genes; global transcription is assessed by measuring nascent mRNA during growth of oocytes from EAFs.
In this study, we examine the effect of Mela and Asta supplementation in the IVG medium for growing oocytes at varying concentrations and in different combinations to explore the role of these antioxidants in oocyte growth. We evaluated their effects on porcine oocyte viability and the expression of specific genes using real-time PCR (RT-PCR), while also measuring nascent mRNA and Me-H3-K4 to assess global transcription. We found that supplementation with combined Mela and Asta at appropriate concentrations during IVG helps growing oocytes attain a quality nearly equivalent to fully grown oocytes isolated from LAFs.
Methods
All the reagents and chemicals used in this research were ordered from Sigma Chemical Co. (St. Louis, Mo, USA), unless otherwise stated.
Ethics statement
The treatment of porcine ovaries used in this study followed the guidelines of the International University–Vietnam National University, Ho Chi Minh City, Viet Nam.
Oocyte cumulus granulosa cell complex (OCGC) collection
Porcine ovaries were collected from local slaughterhouses and transported to the laboratory within two hours in phosphate-buffered saline (PBS; Dulbecco), supplemented with 100 IU penicillin and 100 mg/ml streptomycin. The SAFs (2–3 mm) and the LAFs (4–6 mm) were dissected from the ovaries. To collect EAFs, the ovarian cortex was sectioned into slices 1–2 mm thick using a surgical blade (No. 21). These slices were then placed under a microscope to dissect using a surgical blade (No. 11). After dissection, the follicles were washed in 25 mM HEPES-buffered TCM-199 containing 0.1% polyvinyl alcohol. The follicles were then placed in HEPES and opened using an 18-gauge needle under a microscope. Healthy OCGCs derived from these follicles, characterized by multilayered cumulus and granulosa cells with uniform ooplasm, were selected for the experiment.
Agarose gel preparation and coating dish for culturing
Agarose gel was used to mimic the in vivo microenvironment of the porcine ovarian matrix. To prepare a 1.5% agarose solution, mix low-gelling-temperature agarose (A9539) powder with PBS at a concentration of 1.5% (w/v), heat until fully dissolved, then transfer the hot solution to 1.5 mL centrifuge tubes and store at 4°C. For coating a 96-well dish, reheat the stored agarose gel in boiling water for 5 minutes to remelt it, then apply 30 µL of the molten solution to the bottom of each well. The agarose hardens at room temperature for 10 minutes, and before use, the equilibrated medium is replaced with fresh medium8.
growth (IVG) with antioxidant supplementation
The OCGCs from EAFs were transferred to an IVG medium and cultured under different IVG conditions for 4 days in an incubator with 5% CO in humidified air at 38.5°C. Every well was filled with 120 µL IVG medium and covered with 50 µL mineral oil. Each well could culture 3–5 OCGCs.
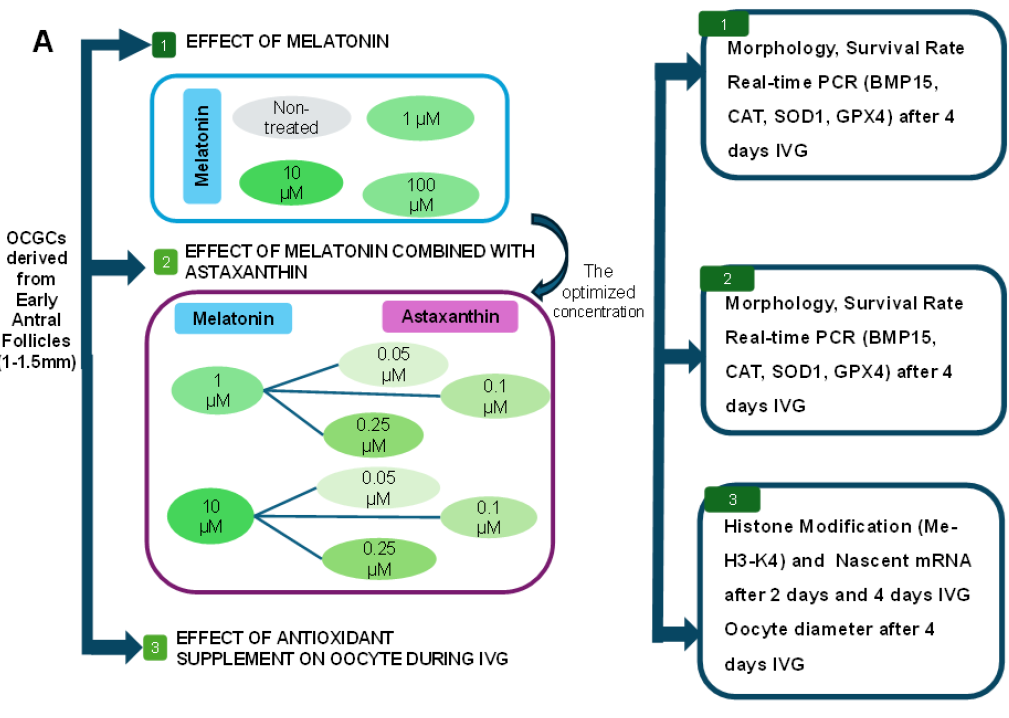
The basic IVG medium contained α-MEM supplemented with 3% polyvinylpyrrolidone, 5% follicle fluid, 10% fetal bovine serum, 0.91 mM NaPy, 3 mM hypoxanthine, and 1 µg/mL estradiol. This study was divided into two main experiments, with each IVG culture medium supplemented with different concentrations of antioxidants. In the first experiment, groups of Mela were divided into three different concentrations (1 µM/mL, 10 µM/mL, and 100 µM/mL) to determine the optimal concentration for the next experiment. In the next experiment, Asta was combined at three different concentrations (0.05 µM/mL, 0.1 µM/mL, and 0.25 µM/mL) with the optimal concentration of Mela.
The OCGCs were cultured in a dish coated with agar. Half of the culture medium was replaced after 2 days. After IVG, the survival rate of OCGCs (n = 54) was evaluated by calculating the percentage of healthy cultured OCGCs. Oocyte diameter (n = 25) was measured after removing the surrounding cells, and both horizontal and vertical dimensions were recorded using a digital microscope.
mRNA extraction and real-time PCR
The mRNA extraction and RT-PCR were performed on the same day to prevent mRNA degradation. According to the manufacturer's instructions, total mRNA was extracted from samples (10–15 oocytes) using the Dynabeads™ mRNA DIRECT™ Kit (Invitrogen, Thermo Fisher Scientific). RT-PCR was performed using the Applied Biosystems™ Power SYBR™ Green RNA-to-CT™ 1-Step Kit (Invitrogen, Thermo Fisher Scientific), and the relative abundance of target mRNAs was examined with specific primers. Act- was used as the reference gene. The primer sequences were listed in
-
Stage 1: Double-stranded DNA synthesis at 48.0°C for 30 minutes and lid heating at 95.0°C for 10 minutes.
-
Stage 2: 45 cycles of denaturation at 95.0°C for 15 seconds, primer annealing, and extension at 60.0°C for 45 seconds.
The sequence of PCR primers
|
Genes |
Primer sequence (5'-3') |
Product size |
|---|---|---|
|
Act- |
Forward: ATCGTGCGGGACATCAAGGA |
179bp |
|
|
Reverse: AGGAAGGAGGGCTGGAAGAG | |
|
BMP15 |
Forward: ACCATGGTGAGGCTGGTGAG |
178bp |
|
|
Reverse: CATGGCAGGAGAGGTGGAAG | |
|
CAT |
Forward: ACGTTGGAAAGAGGACACCC |
137bp |
|
|
Reverse: TCCAACGAGATCCCAATTACCA | |
|
SOD-1 |
Forward: CACTGTGTACATCGAAGATTCTGTGA |
97bp |
|
|
Reverse: GCCCAAGTCATCTGGTTTTTCA | |
|
GPX4 |
Forward: ACGGGCACATGGTGAACCT |
85bp |
|
|
Reverse: ACCTCCGTCTTGCCTCATTG |
Immunofluorescence staining and nascent mRNA detection
Each group of cultured oocytes was washed twice in an electro-permeabilization (EP) medium containing 0.25 M D-glucose, 100 µM CaCl₂, 100 µM MgSO and 0.1% PVP. They were then transferred into a drop of EP medium supplemented with 10 mM BrUTP for 2 minutes. The oocytes were placed in 20 µL of EP medium containing 10 mM BrUTP between two parallel stainless steel electrodes in a chamber. Electro-permeabilization was conducted with one direct current pulse at 30 V and 80 µs. Two minutes post-permeabilization, the oocytes were cultured in IVG medium for 1 hour at 38.5°C with 5% CO. Oocytes were fixed and immunostained, as described in a previous study36. Indirect immunofluorescence detected newly synthesized transcripts incorporating BrUTP and histone methylation H3K4 (Me-H3-K4). The primary antibodies used include mouse monoclonal anti-bromodeoxyuridine (6 µg/mL, Roche-1117037600) and rabbit anti-methyl-histone H3K4. The secondary antibodies, Alexa-Fluor-568-labeled goat anti-mouse IgG and Alexa-Fluor-488-labeled goat anti-rabbit IgG, were used. DNA was counterstained with 2 µg/mL 4,6-diamidino-2-phenylindole (DAPI) for 30 minutes. The levels of Me-H3-K4 expression and BrUTP were analyzed based on fluorescence intensity using the Nikon analysis program. Fluorescence intensity within each nucleus was measured across five different regions, and the cytoplasmic background value was subtracted. The resulting value was then multiplied by the nuclear volume to estimate the total fluorescence for the nucleus. Due to the limited sample size, no statistical analysis could be performed.
Statistical analysis
Each treatment was replicated at least 3 times. Descriptive statistics were performed to assess normality and homogeneity of variance prior to parametric analyses, using skewness values as part of the evaluation. One-way ANOVA was performed with the Statistical Package for Social Sciences (SPSS) version 20, with a significance level of p < 0.05. Data were reported as the mean ± standard error of the mean (SEM). RT-PCR data were analyzed using the Log2FC method, with statistically significant differences at p < 0.05. Based on the Log2FC method, the non-treated group was used as the reference. Therefore, the expression on the graph shows the increase or decrease relative to the non-treated group.

Experimental design and assessment of oocyte-cumulus-granulosa-complexes (OCGCs) supplemented with Mela after IVG. A) Experimental design: Experiment 1: OCGCs collected from EAF were cultured in different concentrations of Mela (1 μM, 10 μM, 100 μM) supplementation; morphology, survival rate, and gene expression were assessed after 4 days. Experiment 2: OCGCs were cultured in different concentrations of combined Mela (1 μM, 10 μM) and Asta (0.05 μM, 0.1 μM, 0.25 μM) supplementation; morphology, survival rate, and gene expression were assessed after 4 days. Experiment 3: Immunofluorescent staining of oocyte after IVG with different concentrations of melatonin groups and combined Mela (1 μM, 10 μM) and Asta (0.05 μM, 0.1 μM, 0.25 μM) groups of Methylation Histone H3K4 (Me-H3-K4) and nascent mRNA on 2 and 4 days IVG and oocyte diameter were assessed after 4 days. Effect of Mela (1 μM, 10 μM, 100 μM) on oocyte derived from EAF. B) Morphology assessment of the OCGCs was checked on days 0 and 4 in IVG. C) The survival rate of growth oocyte after 4 days of IVG. D) RT-PCR results of marker gene in OCGCs cultured with Mela (1 μM, 10 μM, 100 μM) supplement. Data are presented as the mean ± S.E.M. a-c values without a common superscript differed significantly (P < 0.05). Bars = 150 μm
Results
Effects of melatonin on the quality of OCGCs after 4 days of IVG
After IVG culture, antrum formation in OCGCs did not show significant differences between the groups supplemented with Mela (Figure 1B). Nevertheless, Mela positively affected oocytes and granulosa cells, improving survival after 4 days of IVG. The OCGCs supplemented with Mela 10 μM had the highest survival rate, yet no significant differences were observed among the treatment groups (Figure 1C). Based on gene expression studies, Mela slightly promoted expression in growing oocytes (Figure 1D). In addition, Mela enhanced the expression of the and genes, thereby increasing antioxidant enzyme activity and reducing the effects of ROS. However, the differences between each treatment group were negligible, resulting in nearly identical oocyte qualities among the treatment groups.

Impact of combined Mela and Asta supplementation on the quality of oocyte-cumulus-granulosa complexes (OCGCs) after IVG. Effect of Mela 1 mM + Asta on growing oocytes derived from EAFs. A) Morphology assessment of the OCGCs was checked on days 0 and 4 in IVG; B) The survival rate of growth oocyte after 4 days IVG; C) RT-PCR results of OCGCs cultured with Mela 1 µM + Asta supplement after 4 days IVG. Effect of Mela 10 µM + Asta on oocyte derived from EAF; D) Morphology assessment of the OCGCs was checked on days 0 and 4 in IVG; E) The survival rate of growth oocyte after 4 days IVG; F) RT-PCR results of OCGCs cultured with Mela 10 µM + Asta supplement after 4 days IVG. Data are presented as the mean ± S.E.M. a-c values without a common superscript differed significantly (P < 0.05), Bars = 150 µm.
Impact of the combined use of melatonin and astaxanthin on the quality of OCGCs following IVG after 4 days
The addition of combined Mela 1μM + Asta did not significantly affect antrum formation after 4 days of IVG (Figure 2A). However, supplementation with combined Mela 10 μM + Asta reduced the ability to form an antrum after the IVG stage (Figure 2D). The combined supplementation of Mela 1 μM + Asta significantly increased the oocyte survival rate after the IVG stage. Nevertheless, no significant differences were detected among the treatment groups (Figure 2B). Similarly, the combined Mela 10 μM + Asta supplementation increased survival after 4 days of the IVG stage (Figure 2E). However, the quality of oocytes after 4 days of culture showed significant differences. In this case, supplementation with combined Mela 1 μM + Asta may enhance the expression of specific genes. In contrast, the combined Mela 10 μM + Asta groups generally decreased the expression of most of the genes studied (Figure 2C, F). OCGCs supplemented with combined Mela 1 μM + Asta 0.05 μM showed the highest quality after 4 days of IVG, yielding results comparable to fully grown oocytes (LAF). In contrast, groups supplemented with higher concentrations of Mela and Asta exhibited a significant reduction in quality. Both combined Mela 1 μM + Asta and Mela 10 μM + Asta groups demonstrated an increase in expression; however, it was still lower than LAF. OCGCs supplemented with combined Mela 1 μM + Asta showed increased expression of and , while a decrease was observed in the combined Mela 10 μM + Asta group. Among the treatment groups, the combined Mela 1 μM + Asta 0.05 μM group exhibited the highest expression for all genes studied. Therefore, supplementation with combined Mela 1 μM + Asta 0.05 μM significantly increased the oocyte survival rate and quality after IVG.

Effects of Mela supplementation on methylation of histone H3 and oocyte diameter after 2 and 4 days of IVG. A) Immunofluorescent staining of four groups of IVG porcine oocytes derived from early antral follicles (1–1.5 mm) supplemented with Mela (1 μM, 10 μM, 100 μM) after 2 days IVG. Methylation of Histone H3 at lysine 4 (Me–H3-K4) was expressed in green color. The nucleus and its membrane were stained with DAPI; B) The average Me-H3-K4 in corresponding oocytes in different concentrations of Mela (1μM, 10μM, 100μM) after 2 days IVG; C) Oocyte diameter supplemented with Mela after 2 days IVG; D) Immunofluorescent staining of four groups of IVG porcine oocytes derived from early antral follicles (1–1.5 mm) supplemented with Mela (1 μM, 10 μM, 100 μM) after 4 days IVG. Methylation of Histone H3 at lysine 4 (Me–H3-K4) was expressed in green color. The nucleus and its membrane were stained with DAPI; E) Oocyte diameter supplemented with Mela after 4 days IVG. Data are presented as the mean ± S.E.M. a-d values without a common superscript differed significantly (P < 0.05). Bars = 30 μm
Effect of antioxidant supplementation on histone modification of OCGCs and oocyte diameter after 2 and 4 days of IVG
SAF is the preceding stage of LAF; therefore, growing oocytes isolated from SAF must undergo a pre-IVM phase to attain competence equivalent to that of growing oocytes isolated from LAF5. Since the growing oocytes isolated from EAF are of a size comparable to those isolated from SAF, SAF is used for comparative evaluation after 2 days of IVG (Figure 3C). After 2 days of IVG, oocytes supplemented with Mela increased Me-H3-K4 expression compared to the non-treated group (Figure 3B). After 4 days of IVG, Me-H3-K4 levels decreased in all treatment groups as well as in the non-treated group. In Figure 3D, Me-H3-K4 is observed to be concentrated only in the heterochromatin ring, indicating the repression of transcriptional activities. This suggests that the addition of antioxidants does not lead to abnormalities in chromosome configuration26. Therefore, the evaluation of Me-H3-K4 was only performed at IVG day 2. Due to the increase in gene activity, a significant rise in oocyte size was also observed after 4 days of IVG (Figure 3E). Although oocytes exhibited a considerable increase in size following IVG, their diameter remained smaller than that of LAF. This suggests that Mela supplementation during IVG can enhance oocyte quality; however, it has not yet reached the level of LAF.

Effects of combined Mela and Asta on methylation of histone H3 after 2 and 4 days of IVG. A) Immunofluorescent staining of nine groups of IVG porcine oocytes derived from early antral follicles (1–1.5 mm) supplemented with Mela 1μM + Asta (0.05 μM, 0.1 μM, 0.25 μM), Mela 10 μM + Asta (0.05 μM, 0.1 μM, 0.25 μM) after 2 days IVG. Methylation of Histone H3 at lysine 4 (Me–H3-K4) was expressed in green color. The nucleus and its membrane were stained with DAPI; B) The average Me-H3-K4 in corresponding oocytes in different concentrations of combined Mela 1 μM + Asta (0.05 μM, 0.1 μM, 0.25 μM); C) The average Me-H3-K4 in corresponding oocytes in different concentrations of combined Mela 10 μM + Asta (0.05 μM, 0.1 μM, 0.25 μM). Bars = 30 μm
The groups supplemented with the combined Mela 1 μM + Asta 0.05 μM and the combined Mela 10 μM + Asta 0.05 μM showed an increase in Me-H3-K4 expression compared to the other groups (Figure 4B, C). However, both the combined Mela 1 μM + Asta 0.05 μM and combined Mela 10 μM + Asta 0.05 μM groups showed lower intensity than SAF. Therefore, while the oocytes cultured for 2 days had a size similar to SAF, their quality was still lower than that of SAF.

Effect of antioxidant supplementation on nascent mRNA synthesis in oocytes after IVG. A) Immunofluorescent staining of two groups of IVG porcine oocytes derived from early antral follicles (1–1.5 mm) supplemented with Mela 1 μM + Asta 0.05 μM, Mela 10 μM + Asta 0.05 μM. BrUTP (nascent mRNA) was expressed in red color. The nucleus and its membrane were stained with DAPI; B) The average nascent mRNA in corresponding oocytes in different supplemented with Mela 1 μM + Ax 0.05 μM group, Mela 10 μM + Asta 0.05 μM group, and non-treated group after 2 days IVG; C) Oocyte diameter after 4 days IVG. Data are presented as the mean ± S.E.M. a-d values without a common superscript differed significantly (P < 0.05). Bars = 30 μm
The transcriptional activity of the oocytes supplemented with the combined Mela 1 μM + Asta 0.05 μM was higher, as indicated by the greater BrUTP intensity compared to the other two groups, although the combined Mela 10 μM + Asta 0.05 μM group showed a trend towards higher intensity than the non-treated group (Figure 5A, B). Therefore, supplementation with combined Mela 1 μM + Asta 0.05 μM enhances global transcription, improving oocyte quality through increased mRNA storage. Because global transcription only occurs during the FC/SC stage, by day 4 the oocytes have reached the GV stage, where transcription activity has ceased. Therefore, the assessment of global transcription cannot be performed on day 4. Corresponding to the increase in global transcription, the groups supplemented with appropriate antioxidants also showed a significant increase in size compared to the non-treated group. Among these, the combined Mela 1 μM + Asta 0.05 μM group resulted in a notable size increase compared to the other groups, although it was still smaller than LAF (Figure 5C). Therefore, antioxidant supplementation improved both oocyte quality and size, but it has not yet reached the level of LAF.
Discussion
Our study reported that supplementation with the combination of Mela and Asta at appropriate concentrations improved oocyte quality by increasing Me-H3-K4 and global transcriptional activity of growing oocytes after 2 days of IVG. Finally, there was a significant increase in the expression of , an important folliculogenesis regulator gene, as well as antioxidant genes (), thereby reducing the negative effects of ROS on oocytes after IVG. Our culture system supported the growth of oocytes isolated from EAFs to achieve a size and quality equivalent to fully grown oocytes isolated from LAF.
Mela has been shown to mediate its effects on oocytes through MT1 and MT2 membrane receptors, binding to melatonin receptors in the plasma membrane and binding to intracellular proteins such as calmodulin and calreticulin, which helps increase the follicle survival rate13. It has proven effective in IVM culture by stimulating the resumption of meiosis37. Additionally, Mela has been demonstrated to improve granulosa cell quality and mitochondrial activity and to reduce cathepsin B activities31, 38, 39, thereby enhancing oocyte quality. This is consistent with the significant increase in oocyte diameter and marker gene activity (Figure 1D, Figure 3E). Although the differences in marker gene activity were not substantial compared to the non-treated group, a significant improvement in oocyte size was observed in the Mela-supplemented groups. However, OCGCs supplemented with 100 µM Mela exhibited a tendency toward reduced oocyte size compared to those treated with 1 µM and 10 µM Mela. This suggests that while 100 µM Mela still has some positive effects, its adverse impacts begin to emerge, leading to a reduced overall efficacy compared to lower concentrations.
Asta neutralizes reactive oxygen, nitrogen, sulfur, and carbon species by donating electrons or forming stable bonds with free radicals to inactivate them. Asta also induces antioxidant genes and suppresses apoptotic genes in bovine embryos40. Asta may prevent glutamate-induced apoptosis by preserving redox balance and suppressing glutamate-triggered calcium influx and endoplasmic reticulum stress41. It is reported that Me-H3-K4 is highly enriched at transcription start sites, and its presence in enhancers has also been linked to increased transcriptional activity42. Thus, increased Me-H3-K4 was associated with increased gene expression. Moreover, combining both Mela and Asta in IVG is promising due to their different mechanisms of action. We found that combining Asta and Mela at optimal concentrations increased Me-H3-K4 levels and global transcription activity, resulting in improved oocyte quality after IVG. This also significantly improved the survival rate compared to the non-treated group.
Following a 4-day IVG culture, growing oocytes derived from EAFs exhibit chromatin morphology and oocyte diameter similar to those of fully grown oocytes derived from LAFs. Gene expression studies on oocytes supplemented with Mela alone showed a minimal increase compared to the non-treated group. OCGCs cultured in IVG medium supplemented with Mela and Asta also exhibited a significant increase in CAT activity, similar to supplementation with both ascorbic acid and resveratrol43 or coenzyme Q10, which increased antioxidant gene activities44. In mammals, BMP15 is known to play a role in oocyte maturation and cholesterol biosynthesis, as well as enhancing oocyte competence and promoting early embryo development45. Interestingly, combinatory supplementation of astaxanthin and melatonin at lower concentrations demonstrated enhanced BMP15 activity levels in growing oocytes. Similarly, supplementation with Eugenol resulted in significantly higher levels46. Therefore, the significant increase in and antioxidant gene activities contributes to a notable improvement in oocyte quality after IVG. Consequently, the supplementation of astaxanthin at a concentration of 0.05 and melatonin at a concentration of 1 during the IVG culture emerges as the most optimal culture condition.
However, supplementation with high concentrations of Mela led to a significant increase in ROS, consequently reducing oocyte quality47, 48. This is consistent with the significant reduction in the activity of antioxidant genes and BMP15 gene expression (Figure 2F). Moreover, a notable decrease in oocyte size was observed compared to the group supplemented with antioxidants at appropriate concentrations. While the supplementation of 10 µM Mela and Asta did not directly induce oocyte death, it still exhibited negative effects on oocyte quality. This has been demonstrated, as supplementation with high concentrations of Kaempferol and ascorbic acid resulted in lower oocyte quality compared to supplementation with either compound alone49. However, due to certain limitations of the study, it was not possible to clearly distinguish between healthy oocytes and those initiating the apoptosis pathway. As a result, it remains unclear whether the combination of Mela and Asta at inappropriate concentrations truly triggers apoptosis at IVG day 4.
This suggests that excessive supplementation of antioxidants may lead to the disruption of cellular metabolism. When Asta was supplemented with 1 µM Mela, enhanced transcription and Me-H3-K4 levels were observed during 2 days of IVG. It can be concluded that the beneficial effects of Asta are obtained only when combined with lower concentrations of Mela, resulting in improved oocyte quality and overall outcomes in culture. Although OCGCs have been shown to improve quality through evaluations conducted after IVG, further in-depth assessment is necessary at the pre-implantation stage. This would provide a better understanding of mRNA accumulation throughout the IVG process, which could contribute to improving embryo quality. Additionally, this study has not yet evaluated the concentrations of ROS-related compounds such as superoxide, hydrogen peroxide, hydroxyl ions, peroxyl radicals, and hypochlorite ions. Moreover, since the method for assessing nascent mRNA has not been fully optimized, the oocyte survival rate after BrUTP incorporation remains low, resulting in a limited sample size for evaluation. Therefore, additional methods should be implemented to further assess nascent mRNA synthesis capacity.
Conclusions
Growing oocytes isolated from EAFs can attain competence nearly equivalent to fully grown oocytes isolated from LAFs when cultured in our optimal IVG culture systems. Supplementation of antioxidants at appropriate concentrations supports increases in oocyte size, survival rate, and quality by significantly enhancing Me-H3-K4 intensity, global transcription (nascent mRNA), expression, as well as antioxidant gene expression ().
Abbreviations
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 4′,6-diamidino-2-phenylindole (DAPI), alpha minimum essential medium (α-MEM), analysis of variance (ANOVA), astaxanthin (Asta), beta-actin (ActB), bone morphogenetic protein 15 (BMP15), bromouridine triphosphate (BrUTP), carbon dioxide (CO₂), catalase (CAT), early antral follicles (EAFs), electro-permeabilization (EP), fetal bovine serum (FBS), filamentous/stringy chromatin (FC/SC), germinal vesicle (GV), glutathione (GSH), glutathione peroxidase 4 (GPX4), histone H3 lysine 4 methylation (Me-H3-K4), in vitro growth (IVG), in vitro maturation (IVM), large antral follicles (LAFs), log2 fold change (Log2FC), melatonin (Mela), melatonin receptor types 1 and 2 (MT1/MT2), nuclear factor erythroid 2-related factor 2 (NRF2), oocyte cumulus granulosa cell complexes (OCGCs), ovarian tissue cryopreservation (OTC), oxidative stress (OS), phosphate-buffered saline (PBS), polyvinylpyrrolidone (PVP), pre-antral follicles (PAFs), quantitative polymerase chain reaction (qPCR), reactive oxygen species (ROS), Real-time polymerase chain reaction (RT-PCR), small antral follicles (SAFs), sodium pyruvate (NaPy), standard error of the mean (SEM), Statistical Package for the Social Sciences (SPSS), superoxide dismutase 1 (SOD1), and tissue culture medium-199 (TCM-199).
Acknowledgments
We would like to express our heartfelt gratitude to the International University – National University HCMC for providing an excellent research environment in the Cell Reprogramming Laboratory. We extend our thanks to the slaughterhouse Vissan, Tan Binh, and Ut Hao for supplying pig ovaries for research.
Author’s contributions
L.C.T and L.D.T.P designed the experiments, analyzed, investigate, writing – original draft. P.T.D and N.L.B.L and L.B.A.M and C.D.S.P and N.N.T.V and N-T.N: investigated, performed, and analyzed the experiments. N.V.T and H-T.B: project administration, methodology, discussed the results, writing – review & editing. All authors read and approved the final manuscript.
Funding
This research is funded by Vietnam National University HoChiMinh City (VNU-HCM) under grant number B2022-28-01.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

