Non-Invasive Prenatal Testing: Advances, Applications, and Limitations in Prenatal Screening
- Central Laboratory, Qingdao Municipal Hospital, Qingdao, Shandong, China
- Clinical Laboratory, Qingdao Municipal Hospital, Qingdao, Shandong, China
Abstract
Non-invasive prenatal testing (NIPT) is a prenatal screening technology based on the analysis of cell-free fetal DNA (cfDNA) detected in maternal peripheral blood. It offers high detection efficiency for common chromosomal aneuploidies, such as trisomy 21 (T21), trisomy 18 (T18), trisomy 13 (T13), and sex chromosome aneuploidies (SCA). Additionally, NIPT has expanded to include the screening of subchromosomal microdeletions and microduplications, single-gene genetic diseases, and has even demonstrated certain diagnostic value for placental-derived complications during pregnancy. However, some of the problems it presents, such as technical limitations and ethical or psychological issues, cannot be overlooked. This article reviews the advancements and limitations of NIPT in prenatal screening.
Introduction
Since the 1980s, prenatal testing has significantly contributed to reducing congenital disabilities and improving population health. Traditional prenatal screening techniques include ultrasound examination, serological screening, and chromosome karyotype analysis. Nonetheless, these approaches have notable limitations: ultrasound is limited by weak penetration and image distortion, leading to misdiagnosis or missed cases; serological screening has relatively low accuracy; and invasive procedures like amniocentesis and cord blood puncture carry risks of intrauterine infection and miscarriage.
In recent years, high-throughput sequencing based on cell-free DNA (cfDNA), also referred to as non-invasive prenatal testing (NIPT), has emerged as a powerful tool for detecting fetal chromosomal aneuploidy and has gradually become an essential component of prenatal screening. Moreover, advances in NIPT have expanded its applications to include prenatal screening of subchromosomal microdeletions and microduplications, single-gene genetic diseases, and pregnancy complications.
However, any emerging technology inevitably introduces some negative consequences. Therefore, this article summarizes the advancements in prenatal screening and analyzes the limitations of NIPT, as well as the associated ethical and psychological implications for pregnant women.
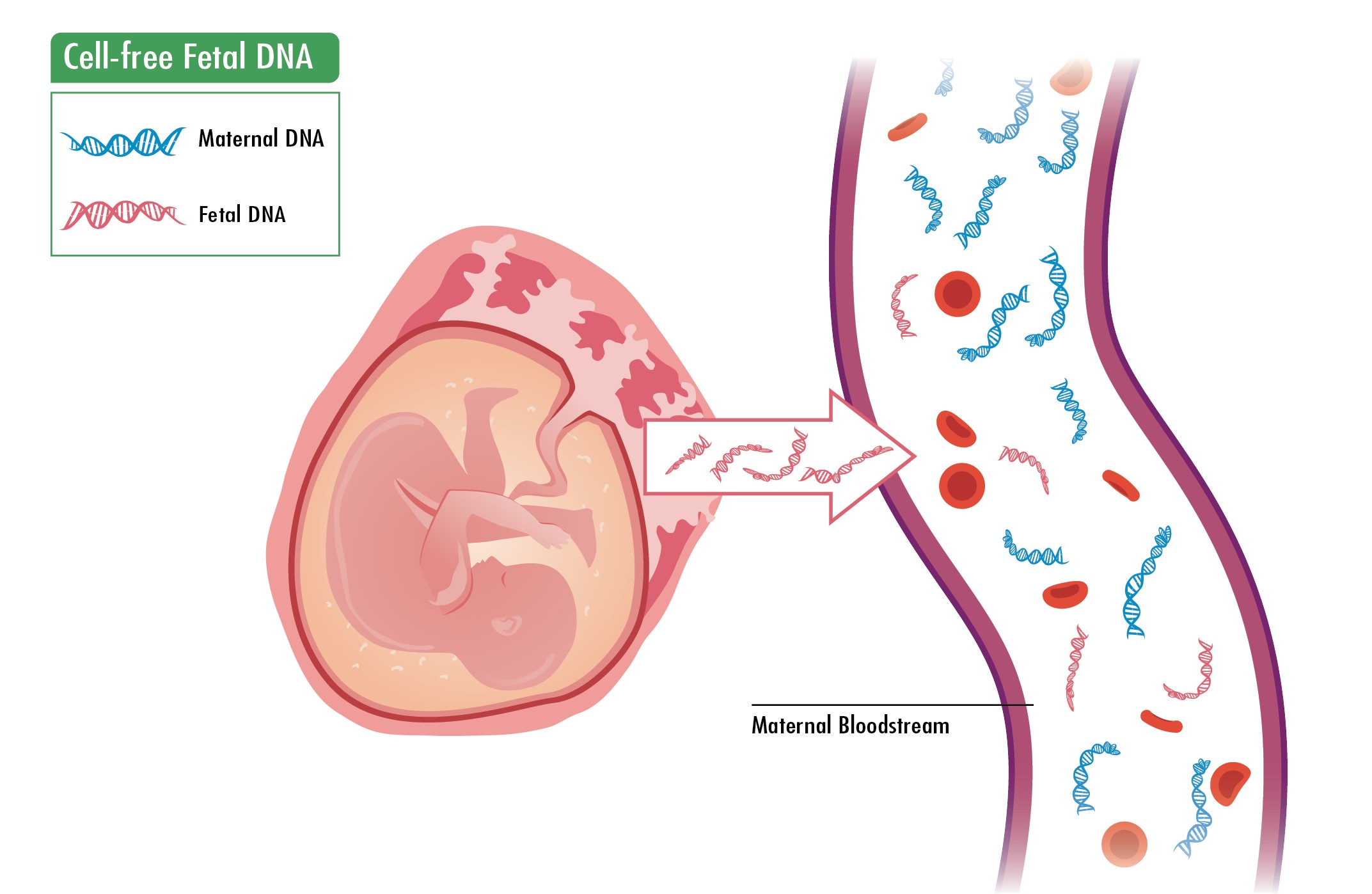
Source and detection principle of cfDNA in maternal peripheral blood
As early as 1997, Lo .1 detected fetal Y chromosome DNA in maternal peripheral blood, confirming the existence of cell-free DNA (cfDNA) and establishing the theoretical foundation for non-invasive prenatal testing (NIPT). Subsequent studies revealed that cfDNA concentration in maternal peripheral blood increases with gestational age and can be detected as early as the 7 week of pregnancy2. CfDNA is primarily derived from placental trophoblasts and maternal hematopoietic cells3, 4, 5, consisting of small fragments approximately 100–200 base pairs in length, which account for 5%–30% of the total cfDNA in maternal blood. After childbirth, cfDNA rapidly degrades, becoming undetectable within 2 hours, with an average half-life of 16.3 minutes6, 7, 8. For a long time, the low concentration of fetal cfDNA and high background noise from maternal DNA hindered its application in prenatal screening. However, with the advancement of next-generation sequencing (NGS), this limitation has been overcome. NGS offers high throughput, enabling large-scale genomic sequencing. This technology can simultaneously analyze hundreds of thousands to millions of DNA sequences, allowing for whole-genome sequencing of samples. By quantifying changes in the number of these DNA fragments, bioinformatics analysis can detect fetal chromosomal abnormalities. Due to its efficacy and accuracy, NGS-based cfDNA analysis has been adopted for clinical prenatal testing.
Clinical application of NIPT
Detection of Fetal T21, T18, T13, and SCA
Non-invasive prenatal testing (NIPT) is highly sensitive and specific for detecting aneuploidies in singleton fetuses on chromosomes 21, 18, and 13. A meta-analysis by Gil MM .9 of over 200,000 pregnant women with singleton fetuses found that NIPT had detection rates of 99.7% for T21, 97.9% for T18, and 99.7% for T13, with false-positive rates of 0.04% for each. However, current reports on NIPT’s accuracy vary. Some studies report a positive predictive value (PPV) of 83% to 92.9% for NIPT in detecting sex chromosome aneuploidies (SCA) in singleton pregnancies10, 11, 12, while others report a PPV as low as 53.1%13.
Additionally, with the rising incidence of infertility, the number of twin or multiple pregnancies resulting from assisted reproductive technology has increased annually. Studies have evaluated NIPT’s accuracy in detecting chromosomal abnormalities in twins, showing that cell-free DNA (cfDNA) testing for T21, T18, and T13 in twin pregnancies yields results comparable to those in singleton pregnancies14, 15, 16. However, the limited number of SCA cases in these studies precludes an accurate assessment of cfDNA’s predictive value for these anomalies.
Detection of Autosomal Abnormalities and Subchromosomal Microdeletions/Microduplications
With the continuous development of sequencing technologies and advances in clinical settings, international and domestic consensus guidelines have expanded the applications of non-invasive prenatal testing (NIPT). These now include screening for additional chromosomal numerical abnormalities and microdeletion/microduplication syndromes. In published studies, researchers found that a high-risk NIPT result for chromosomes 2, 9, and 22 could be confirmed as fetal trisomy or mosaic trisomy through follow-up tests such as fluorescence in situ hybridization (FISH) and DNA microarray17, 18, 19. Although NIPT has demonstrated potential for detecting other autosomal abnormalities, its application to all chromosomes remains controversial due to ethical concerns, increased psychological burden on pregnant women, and the potential for unnecessary invasive diagnostic procedures.
Microdeletion/microduplication syndromes, caused by chromosomal copy number variations (CNVs), are characterized by complex phenotypes and account for 1–2% of congenital malformations in neonates. The accuracy of NIPT for these conditions depends on factors such as: the proportion of cell-free DNA (cfDNA) in maternal plasma, the size of the fetal microdeletion/microduplication, sequencing coverage, and bioinformatics analysis methods20, 21.
NIPT-plus, an advanced version of NIPT, improves the detection rate of microdeletion/microduplication syndromes by increasing sequencing depth and refining algorithms—without altering clinical procedures, requiring additional blood samples, or extending reporting time22. For example, a study by Xue .23 reported that NIPT-plus achieved an 80% positive predictive value (PPV) for 22q11.2 microduplication syndrome, with PPVs of 75% for DiGeorge syndrome, and 50% for both Prader-Willi/Angelman syndrome and Cri-du-chat syndrome.
Currently, NIPT screening for common trisomies and sex chromosome aneuploidies (SCA) is widely accepted among pregnant women. However, its use for microdeletion/microduplication syndromes remains debated. Opponents argue that the relatively low PPV, high false-positive rate, and uncertain clinical significance of some CNVs create challenges in managing high-risk results. Proponents, however, emphasize that the primary goal of prenatal screening is to reduce the burden of fetal chromosomal abnormalities at birth. Thus, they advocate for including even low-PPV microdeletions/microduplications in screening programs.
Detection of Monogenic Diseases
Single-gene inherited diseases are a significant cause of congenital disabilities, resulting from pathogenic variants in a single gene and affecting approximately 1% of neonates24. Although each individual monogenic disease is rare, collectively, they are prevalent, with over 6,000 distinct disorders of known pathogenesis identified. Their combined incidence is similar to that of chromosomal disorders, with dominant conditions representing more than half of cases25. Affected children often present with severe symptoms and have limited treatment options. Furthermore, since many cases arise from de novo mutations, these children frequently lack a family history of the disease, and their parents usually exhibit normal phenotypes. This makes prenatal diagnosis particularly challenging and often leads to underdetection.
Numerous studies have reported the use of non-invasive prenatal testing (NIPT) for single-gene disorders, including thalassemia, phenylketonuria, and Duchenne muscular dystrophy26, 27, 28. Notably, follow-up studies with invasive diagnostic confirmation have demonstrated strong concordance between NIPT and traditional diagnostic methods. In 2022, a study by Baylor College of Medicine screened over 2,000 individuals for 25 dominant monogenic diseases (involving 30 genes) and reported a positive detection rate of 5.7%, highlighting the clinical utility of NIPT for dominant monogenic disorders29. However, NIPT for fetal single-gene diseases has not yet been widely adopted in clinical practice due to high costs, lengthy turnaround times, and complex bioinformatic analysis.
Prenatal screening for pregnancy complications
Common complications during pregnancy include gestational diabetes mellitus (GDM), intrahepatic cholestasis of pregnancy (ICP), and preeclampsia. These conditions are highly prevalent, yet reliable methods for their early diagnosis remain lacking. Since cell-free DNA (cfDNA) is primarily derived from the placenta—the origin of many pregnancy complications—researchers have explored the potential of cfDNA fragment analysis for molecular detection. This approach has demonstrated promise not only for predicting fetal chromosomal abnormalities but also for assessing the risk of pregnancy complications in the mother.
GDM poses both short- and long-term risks to mothers and fetuses. Affected women face higher risks of polyhydramnios, metabolic disorders, and cesarean delivery, while their fetuses may experience macrosomia, growth restriction, and an elevated lifelong risk of diabetes and obesity. Bauer found an association between elevated cfDNA levels and GDM, suggesting cfDNA’s potential as a predictive marker30.
ICP is characterized by severe pruritus, abnormal liver function, and elevated serum bile acids. This condition has a notable incidence during pregnancy and is associated with premature birth and intrauterine fetal death. A study of 831 pregnant women who received NIPT revealed that maternal cfDNA levels were significantly elevated in ICP cases compared to controls31.
Preeclampsia presents with hypertension, edema, and proteinuria. Its pathogenesis involves reduced uterine-placental blood supply and/or increased fetal-placental demand, leading to a perfusion mismatch. This triggers the release of stress-related factors from the placenta and disrupts the balance between pro-angiogenic placental growth factor and anti-angiogenic soluble fms-like tyrosine kinase-1 (sFlt-1). Kumar . demonstrated that cfDNA levels are higher in preeclamptic women than in healthy controls32. Moreover, early-pregnancy whole-genome sequencing of plasma cfDNA combined with preeclampsia-associated promoter analysis achieved a prediction accuracy of 83%33.
Limitations of NIPT
Limitations of Technology and Clinical Applications
Non-invasive prenatal testing (NIPT) relies on short fragments of cell-free DNA (cfDNA) from the placenta in maternal peripheral blood, typically less than 200 bp in length. These fetal cfDNA signals may be diluted or obscured by maternal background DNA, increasing the risk of false-negative results for microdeletion/microduplication syndromes and monogenic diseases. The accuracy of NIPT is highly dependent on the fetal fraction (the proportion of fetal cfDNA in maternal blood). A low fetal fraction—due to early gestational age (<10 weeks) or maternal obesity (BMI ≥ 30)—increases the risk of false negatives34.
Fetal cfDNA primarily originates from placental trophoblast cells. Discrepancies between the placental and fetal karyotypes (., confined placental mosaicism) can lead to false-positive or false-negative results35. Similarly, in twin pregnancies, an abnormal or "vanishing" twin may cause erroneous NIPT results36. Maternal chromosomal abnormalities—such as sex chromosome chimerism or microdeletions—may also be misattributed to the fetus, leading to false positives37. Additionally, malignant tumors in the mother can release tumor-derived DNA with chromosomal abnormalities, further increasing the risk of false positives38.
Notably, NIPT cannot detect fetal structural abnormalities, such as neural tube defects or cardiac malformations; these still require ultrasound assessment.
Although NIPT is a screening tool, some patients mistakenly believe it can replace diagnostic tests like amniocentesis. Positive NIPT results must be confirmed through chorionic villus sampling (CVS) or amniocentesis. However, CVS carries a risk of misdiagnosis due to placental mosaicism. Furthermore, pregnancies involving embryo donation or surrogacy may complicate result interpretation due to genetic disparities between the mother and fetus. Finally, most NIPT algorithms are optimized for singleton pregnancies, and their accuracy in multiple gestations requires improvement39.
Ethical and Psychological Challenges of NIPT
NIPT may lead to overtreatment, as false-positive results can prompt unnecessary invasive diagnostic procedures, increasing the risk of fetal loss. Conversely, false-negative results may result in medical disputes. Some medical institutions fail to adequately communicate the limitations of NIPT, leading patients to overestimate its accuracy and causing some pregnant women to mistakenly believe that NIPT can detect all birth defects. Furthermore, NIPT can determine fetal sex early, raising concerns about misuse in regions with a strong gender preference.
Beyond considering NIPT’s accuracy, the psychological impact on pregnant women must not be overlooked40. Before testing, pregnant women should carefully weigh the benefits of early detection against potential risks, such as the stress of receiving positive results. High-risk results often trigger acute anxiety or depression, and the decision to undergo invasive procedures (., amniocentesis) may create a dilemma between miscarriage risks and reproductive choices. Additionally, deciding whether to continue a pregnancy upon diagnosing chromosomal abnormalities with mild phenotypes (., XXY, XXX, or XYY) or microdeletion/microduplication syndromes can cause conflicts between partners.
False positives not only pose physical risks through unnecessary invasive tests but may also undermine pregnant women’s trust in the medical system. Conversely, false negatives—where an abnormality is unexpectedly discovered after delivery—may lead to anger or depression in families due to lack of prior warning.
Conclusions
Prenatal screening is particularly important because effective treatments for chromosomal diseases are currently lacking, and the risk of having a child with chromosomal abnormalities increases with maternal age, placing a significant burden on families and society. NIPT is a non-invasive, safe, and relatively painless test that has proven effective in detecting trisomy 21, trisomy 18, trisomy 13, and SCA, leading to widespread public acceptance. However, its application in detecting other chromosomal aneuploidies, fetal chromosome copy number variations, single-gene genetic disorders, and pregnancy complications remains in the exploratory stage, with significant limitations in technical principles, social ethics, and the psychological impact on pregnant women.
In the future, balancing the medical benefits and social risks of NIPT will require technological innovation, standardized management, and ethical guidelines to ensure precise and responsible clinical use.
Abbreviations
BMI (Body Mass Index) is a measure of body fat based on height and weight; bp (base pairs) are units of length for DNA fragments (e.g., 100–200 bp); cfDNA (cell-free DNA) refers to short DNA fragments circulating in the maternal bloodstream, primarily fetal DNA from the placenta in prenatal testing; CNVs (copy number variations) are gains or losses of DNA segments, which can result in microdeletions or microduplications; CVS (chorionic villus sampling) is an invasive prenatal diagnostic procedure that tests placental tissue for genetic abnormalities; FISH (fluorescence in situ hybridization) is a laboratory technique used to detect and localize specific DNA sequences on chromosomes; GDM (gestational diabetes mellitus) is a form of diabetes diagnosed during pregnancy; ICP (intrahepatic cholestasis of pregnancy) is a liver disorder in pregnancy, associated with severe itching and elevated bile acids; NGS (next-generation sequencing) is a high-throughput DNA sequencing technology enabling large-scale analysis, which underlies many NIPT workflows; NIPT (non-invasive prenatal testing) is a prenatal screening method analyzing cfDNA in maternal blood to detect chromosomal abnormalities in the fetus; PPV (positive predictive value) represents the likelihood that a positive test result correctly indicates the presence of a condition; SCA (sex chromosome aneuploidies) are abnormalities in the number of X or Y chromosomes (e.g., Turner syndrome [XO], Klinefelter syndrome [XXY]); sFlt-1 (soluble fms-like tyrosine kinase-1) is an anti-angiogenic factor implicated in preeclampsia; T13 (trisomy 13) refers to an extra copy of chromosome 13; T18 (trisomy 18) is an extra copy of chromosome 18; and T21 (trisomy 21) is an extra copy of chromosome 21 (Down syndrome).
Acknowledgments
None.
Author’s contributions
All authors contributed equally to the conception, literature review, and writing of this manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.

