Revealing the Role of Tumor Budding in Predicting Survival in Colorectal Adenocarcinoma
- Department of Pathology, Hanoi Medical University, Vietnam. 1, Ton That Tung Street, Dong Da District, Ha Noi City, Viet Nam
- Department of Pathology, Hospital of Hanoi Medical University, Vietnam. 1, Ton That Tung Street, Dong Da District, Ha Noi City, Viet Nam
- Department of Pathology, Phenikaa University, Vietnam. Nguyen Trac Street, Ha Dong District, Hanoi, Viet Nam
- Department of Hematology and Cell Diagnostics – National Hospital of Endocrinology, Vietnam. Ngoc Hoi Street, Thanh Tri District, Hanoi City, Viet Nam
- Department of Human Pathology, Kanazawa University Graduate School of Medical Sciences, Japan. 13-1 Takaramachi, Kanazawa, Ishikawa 920-8640, Japan
- Department of Quan Su Pathology, National Cancer Hospital, Vietnam. 43, Quan Su Street, Hoan Kiem District, Ha Noi City, Viet Nam
Abstract
Introduction: Several studies have shown that classical histopathological features (e.g., type, grade, pT stage) are not consistently reliable predictors of survival in colorectal cancer (CRC). Therefore, the present study aimed to assess the value of tumor budding on tissue sections in predicting survival outcomes in patients with CRC.
Methods: Tissue samples were obtained from 107 patients diagnosed with stage I, II, or III CRC at Hanoi Medical University Hospital between 2017 and 2018, who had not undergone preoperative chemoradiotherapy. Using routine hematoxylin and eosin (H&E)-stained sections, tumor budding (Bd) was evaluated following the guidelines established by the International Tumor Budding Consensus Conference (2016). The Kaplan–Meier method was used to estimate overall and disease-free survival, and a Cox proportional hazards model was employed to identify factors independently associated with both outcomes.
Results: Tumor budding grades were identified in 53.3%, 28.0%, and 18.7% of cases for Bd1, Bd2, and Bd3, respectively. The median follow-up duration was 70 months. Overall and disease-free survival decreased with higher Bd levels, with corresponding rates of 98.2%, 80.0%, and 45.0% (p < 0.001) and 94.7%, 80.0%, and 30.0% (p < 0.001) for Bd1, Bd2, and Bd3, respectively. Moreover, Bd correlated significantly with survival, T stage, N stage, TNM stage, lymphovascular invasion, and neural invasion (p < 0.05).
Conclusions: Tumor budding showed a strong association with survival outcomes, lymph node metastasis, and TNM stage. Therefore, it may serve as an independent predictor of survival in patients at stage I, II, or III CRC.
Introduction
Colorectal cancer (CRC) is the most common gastrointestinal malignancy. According to GLOBOCAN 2020, CRC accounts for 10.01% of all new cancer cases worldwide, ranking third behind lung and breast cancers, and contributing to 9.39% of cancer-related deaths—second only to lung cancer. In Vietnam, CRC is the fourth most common cancer, after breast, liver, and lung cancers, with 16,426 reported cases1. Several studies have emphasized the complexity of predicting survival outcomes in CRC and suggested that classical histopathological features, including histological type, histological grade, and pT stage, are neither consistent nor reliable predictors of survival, particularly for signet ring cell carcinoma and mixed adenocarcinoma subtypes.
To minimize potential confounding and remain widely applicable in developing countries, optimal survival predictors should be technically simple, reproducible, and feasible in resource-limited settings. Therefore, we evaluated the prognostic utility of tumor budding (Bd), introduced by the International Tumor Budding Consensus Conference (ITBCC) in 2016. Several studies have shown that Bd can predict lymph node metastasis, disease stage, and survival in patients with gastric2, 3, breast4, and colon cancer5. Our study was conducted to further highlight the value of Bd in predicting survival, lymph node metastasis, and TNM stage in patients with CRC.
Methods
Patients and tumor samples
In this study, we retrospectively examined hematoxylin and eosin (H&E)-stained sections from 107 patients with primary CRC who underwent tumor resection and regional lymphadenectomy at the Department of Pathology of Hanoi Medical University Hospital between January 2017 and December 2018. After surgery, 88 patients subsequently received adjuvant chemotherapy with the FOLFOX-6 regimen. The remaining 19 patients did not receive postoperative chemotherapy because they either presented with early-stage cancer or declined adjuvant treatment. Patients’ clinical and surgical data were extracted from digital records, including age, sex, tumor location, time of diagnosis, number of lymph nodes dissected, and metastatic location (if any). All personal information was coded to ensure anonymity. This study was approved by the Ethics Review Board of Hanoi Medical University (IRB-VN 922) and conducted in accordance with the ethical principles of the 1975 Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study, patient anonymity, and the absence of any additional interventions.
Histological examination
H&E-stained sections were independently reviewed by two pathologists (D.T.L. and N.V.C.) for variables such as tumor size, histologic type, tumor differentiation, invasion, lymphatic invasion, nerve invasion, lymph node status, and Bd. In cases of discrepancy in the histologic results, a third pathologist (N.V.H.) was consulted. A final diagnosis was determined by consensus among all pathologists. All histologic evaluations were performed without prior knowledge of patients’ clinical outcomes. The concordance rate of the three-tier classification between the two pathologists was 99.6%, with a Cohen’s kappa coefficient of 0.993 (P < 0.001), indicating excellent agreement.
Histological type and grade were assigned based on the 2019 World Health Organization histological classification of gastrointestinal tumors6 and the 2017 (eighth) edition of the American Joint Committee on Cancer (AJCC) staging system, which applies only to conventional adenocarcinomas6, 7. The extent of invasion and regional lymph node metastasis were classified according to the eighth edition of the AJCC staging system7.
The procedure for assessing Bd status was performed in five steps as proposed by the 2016 ITBCC5: 1) determine the field area of the 20x microscope objective based on the eyepiece field number diameter; 2) select the H&E-stained section with the highest budding level or “hot spot” in the invasive margin; 3) scan 10 individual fields under medium magnification (10x objective) to identify the chosen “hot spot” area; 4) count the tumor buds in the selected “hot spot” (20x objective); 5) divide the number of buds by the normalization factor to determine the number of tumor buds per 0.785 mm². Bd was classified into three levels according to ITBCC5 recommendations: low-grade (Bd1): 0–4; intermediate-grade (Bd2): 5–9 buds; high-grade (Bd3): 10 or more buds.
Follow-up and outcomes
Overall survival (OS) was defined as the length of time from the start of treatment or diagnosis until death from any cause. Disease-free survival (DFS) was calculated from the time of tumor resection until the time of first recurrence or death from any cause. Recurrence status was determined using imaging and/or histopathology records. Patients who were still alive at the end of the study period were considered censored observations. Patients with incomplete clinical data or those lost to follow-up were excluded from the analysis to ensure data integrity. The follow-up ended on August 31, 2024.
Statistical analysis
Data analyses were conducted using SPSS version 20.0. Pearson’s chi-squared test, Fisher’s exact test, Cramer’s V-test, and Phi were used to compare clinical and histopathological differences among Bd groups. The Kaplan-Meier method was used to calculate both OS and DFS, with survival curves compared using the log-rank test. In the multivariate analysis, the Cox proportional hazards model was applied to identify factors independently associated with OS and DFS. P-values ≤ 0.05 were considered statistically significant.
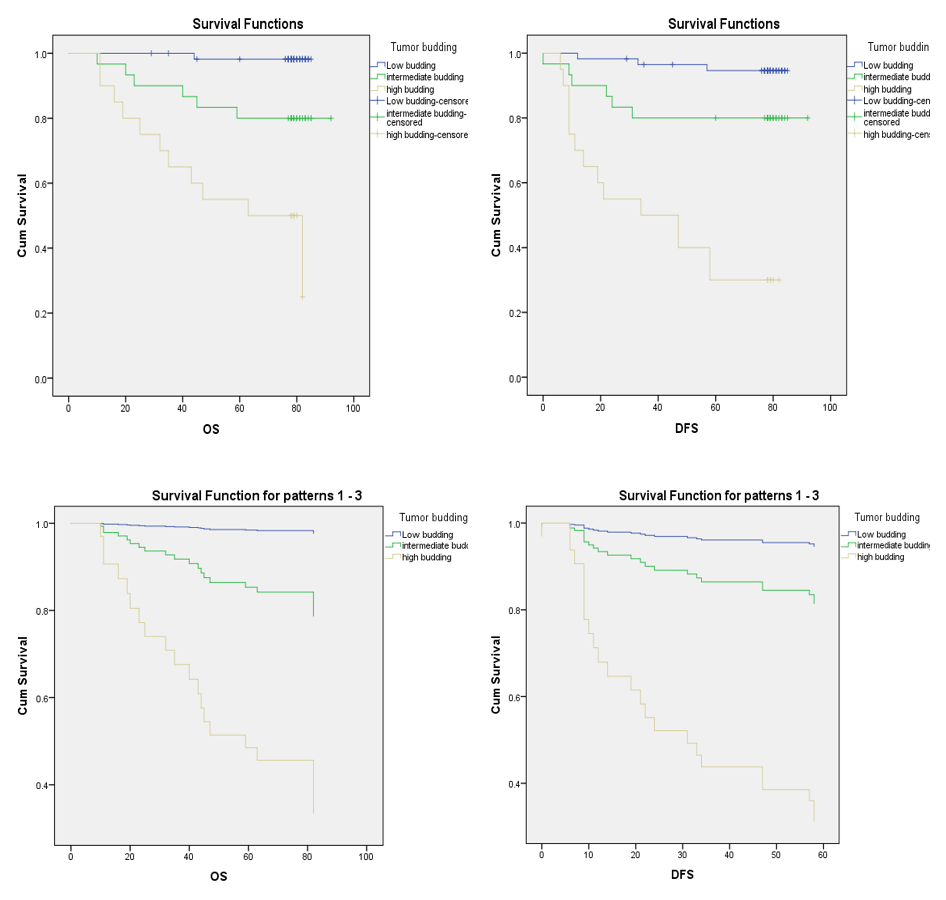
Overall survival and disease-free survival rates for different tumor budding grades in colorectal cancer. The log-rank test demonstrated significant differences among these three survival curves, in both univariate and multivariate analysis (p < 0.001)
Patient clinicopathological characteristics
|
Clinicopathological characteristics |
Number of patients |
Percentage (%) | |
|---|---|---|---|
|
Age group |
<40 |
4 |
3.7 |
|
40-49 |
15 |
14.0 | |
|
50-59 |
16 |
15.0 | |
|
60-69 |
39 |
36.4 | |
|
>=70 |
33 |
30.8 | |
|
Sex |
Male |
59 |
55.1 |
|
Female |
48 |
44.9 | |
|
Location |
Rectum |
33 |
30.8 |
|
Sigmoid colon |
26 |
24.3 | |
|
Left colon |
9 |
8.4 | |
|
Transverse colon |
8 |
7.5 | |
|
Right colon |
24 |
22.4 | |
|
Cecum |
7 |
6.5 | |
|
Tumor differentiation |
Well |
5 |
4.7 |
|
Moderately |
92 |
86 | |
|
Poorly |
10 |
9.3 | |
|
Histopathological type |
Adenocarcinoma |
89 |
83.2 |
|
Mucinous adenocarcinoma |
16 |
15.0 | |
|
Others |
2 |
1.9 | |
|
T stage |
1 |
5 |
4.7 |
|
2 |
14 |
13.1 | |
|
3 |
59 |
55.1 | |
|
4 |
29 |
27.1 | |
|
N stage |
N0 |
62 |
57.9 |
|
N1 |
34 |
31.8 | |
|
N2a |
7 |
6.5 | |
|
N2b |
4 |
3.7 | |
|
TNM stage |
I |
6 |
5.6 |
|
II |
56 |
52.3 | |
|
III |
45 |
42.1 | |
|
Lymphovascular invasion |
No |
65 |
60.7 |
|
Yes |
42 |
39.3 | |
|
Perineural invasion |
No |
68 |
63.6 |
|
Yes |
39 |
36.4 | |
|
Tumor budding |
Low-grade |
57 |
53.3 |
|
Intermediate-grade |
30 |
28.0 | |
|
High-grade |
20 |
18.7 | |
Results
Clinicopathological features and Bd
This study included tissue samples from 107 CRC patients who underwent surgical resection. The mean age of the patients was 63.3 ± 12.7 years (range: 32–88 years), although most patients were aged 60–69 years (36.4%) or over 70 years (30.8%). Males accounted for a higher proportion of patients than females (55.1% . 44.9%, respectively). The most common anatomical site was the rectum (30.8%). Most cases were adenocarcinoma not otherwise specified (83.2%), moderately differentiated (86.0%), and in the pT3 stage (55.1%). The overall rate of lymph node metastasis was 42.1%; metastasis to 1–3 lymph nodes was most frequent (31.8%). Stage I, II, and III tumors accounted for 5.6%, 52.3%, and 42.1% of cases, respectively. The rates of lymphatic and perineural invasion were 39.3% and 36.4%, respectively. Bd1, Bd2, and Bd3 rates were 53.5%, 28.0%, and 18.7%, respectively (
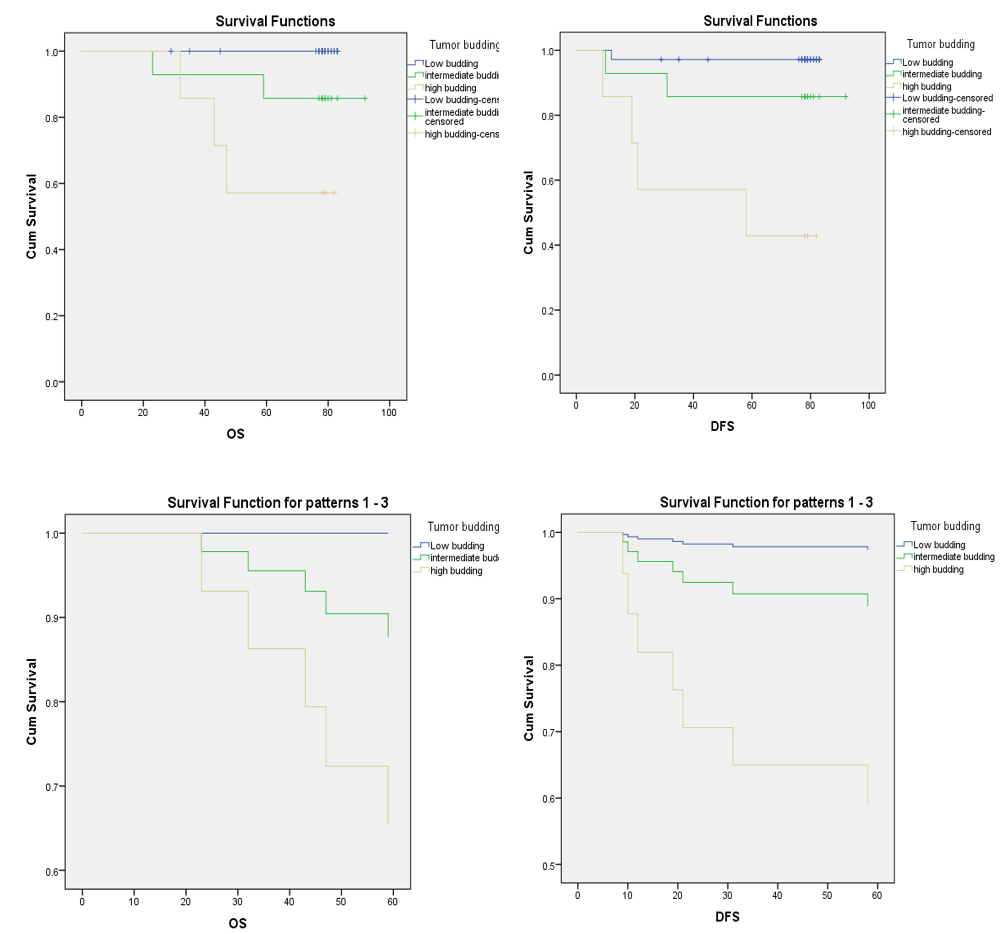
Overall survival and disease-free survival rates of tumor budding grades for stage II colorectal cancer.
Correlation between tumor budding and some histopathological characteristics
|
Histopathological characteristics |
Tumor budding |
p-value | |||
|
Low-grade |
Intermediate-grade |
High-grade | |||
|
pT stage |
pT1 |
4 (80.0) |
0 |
1 (20.0) |
0.021 |
|
pT 2 |
11 (78.6) |
0 |
3 (21.4) | ||
|
pT 3 |
32 (54.2) |
18 (30.5) |
9 (15.3) | ||
|
pT 4 |
10 (34.5) |
12 (41.4) |
7 (24.1) | ||
|
Tumor differentiation |
Well |
5 (100) |
0 |
0 |
0.301 |
|
Moderately |
48 (52.2) |
27 (20.3) |
17 (18.5) | ||
|
Poorly |
4 (40.0) |
3 (30.0) |
3 (30.0) | ||
|
Lymphovascular invasion |
No |
44 (67.7) |
14 (21.5) |
7 (10.8) |
0.001 |
|
Yes |
13 (31.0) |
16 (38.1) |
13 (31.0) | ||
|
Perineural invasion |
No |
48 (70.6) |
13 (19.1) |
7 (10.3) |
<0.001 |
|
Yes |
9 (23.1) |
17 (43.6) |
13 (33.3) | ||
|
N stage |
N0 |
41 (66.1) |
14 (22.6) |
7 (11.3) |
0.009 |
|
N1 |
14 (41.2) |
10 (29.4) |
10 (29.4) | ||
|
N2a |
2 (28.6) |
3 (42.9) |
2 (28.6) | ||
|
N2b |
0 |
3 (75.0) |
1 (25.0) | ||
|
TNM stage |
I |
6 (100) |
0 |
0 |
0.01 |
|
II |
35 (62.5) |
14 (25.0) |
7 (12.5) | ||
|
III |
16 (35.6) |
16 (35.6) |
13 (28.9) | ||
Association of Bd with other outcomes
The mean follow-up period was 70.81 months (maximum: 92 months). At the end of the follow-up period, 85 patients (79.4%) were alive, 23 had relapsed, and 18 had died. Patients with deep invasion, lymph node metastasis, advanced TNM stage, and lymphatic invasion had a significantly higher recurrence rate (p < 0.05) and higher mortality (p < 0.05) than the corresponding reference group. Furthermore, survival was significantly inversely associated with Bd level, with OS rates of 98.2%, 80.0%, and 45.0% (p < 0.001) and DFS rates of 94.7%, 80.0%, and 30.0% (p < 0.001) for Bd1, Bd2, and Bd3, respectively (
OS and DFS were significantly different among Bd groups. Specifically, the mean OS was 84.255 ± 0.739 months in the low-grade Bd1 group and 56.1 ± 6.645 months in the high-grade Bd3 group. Similarly, the DFS in the low-grade Bd1 group was significantly longer than that in the high-grade Bd3 group (82.271 ± 1.625 months vs. 42.05 ± 6.843 months) (
In the multivariate analysis, patients in the Bd3 group had higher risks of recurrence and death than patients in the combined Bd1 and Bd2 group (p < 0.001), with hazard ratios (HRs) for death and recurrence of 45.952 and 20.652, respectively (
Correlation between outcome with some clinicopathological characteristics
|
Clinicopathological characteristics |
Outcome |
p-value |
Recurrence |
p-value | |||
|
Censored |
Event |
No |
Yes | ||||
|
Sex |
Male |
47 (79.7) |
12 (20.3) |
0.312# |
43 (72.9) |
16 (27.1) |
0.157# |
|
Female |
42 (87.5) |
6 (12.5) |
41 (85.4) |
7 (14.6) | |||
|
Age group |
30-39 |
4 (100) |
0 |
0.125* |
4 (100) |
0 |
0.042* |
|
40-49 |
13 (86.7) |
2 (13.3) |
12 (80.0) |
3 (20.0) | |||
|
50-59 |
13 (86.7) |
3 (18.8) |
13 (81.2) |
3 (18.8) | |||
|
60-69 |
36 (92.3) |
3 (7.7) |
35 (89.7) |
4 (10.3) | |||
|
≥70 |
23 (69.7) |
10 (30.3) |
20 (60.6) |
13 (39.4) | |||
|
Location |
Rectum |
28 (84.8) |
5 (15.2) |
0.938* |
26 (78.8) |
7 (21.2) |
0.647* |
|
Sigmoid colon |
22 (84.6) |
4 (15.4) |
21 (80.8) |
5 (19.2) | |||
|
Left colon |
7 (77.8) |
2 (22.2) |
6 (66.7) |
3 (33.3) | |||
|
Transverse colon |
7 (87.5) |
1 (12.5) |
7 (87.5) |
1 (12.5) | |||
|
Right colon |
20 (83.3) |
4 (16.7) |
20 (83.3) |
4 (16.7) | |||
|
Cecum |
5 (71.4) |
2 (28.6) |
4 (57.1) |
3 (42.9) | |||
|
T stage |
1 |
4 (80.0) |
1 (20.0) |
0.038* |
4 (80.0) |
1 (20.0) |
0.203* |
|
2 |
11 (78.6) |
3 (21.4) |
11 (78.6) |
3 (21.4) | |||
|
3 |
54 (91.5) |
5 (8.5) |
50 (84.7) |
9 (15.3) | |||
|
4 |
20 (69.0) |
9 (31.0) |
19 (65.5) |
10 (34.5) | |||
|
Tumor differentiation |
Well |
5 (100) |
0 |
0.493* |
5 (100) |
0 |
0.511* |
|
Moderately |
77 (83.7) |
15 (16.3) |
72 (78.3) |
20 (21.7) | |||
|
Poorly |
7 (70.0) |
3 (30.0) |
7 (70.0) |
3 (30.0) | |||
|
Histopathological type |
Adenocarcinoma |
75 (84.3) |
14 (15.7) |
0.33* |
70 (78.7) |
19 (21.3) |
0.499* |
|
Mucinous adenocarcinoma |
13 (81.2) |
3 (18.8) |
13 (81.2) |
3 (18.8) | |||
|
Others |
1 (50.0) |
1 (50.0) |
1 (50.0) |
1 (50.0) | |||
|
Lymphovascular invasion |
No |
58 (89.2) |
7 (10.8) |
0.062# |
56 (86.2) |
9 (13.8) |
0.029# |
|
Yes |
31 (73.8) |
11 (26.2) |
28 (66.7) |
14 (33.3) | |||
|
Perineural invasion |
No |
60 (88.2) |
8 (11.8) |
0.105# |
58 (85.3) |
10 (14.7) |
0.03# |
|
Yes |
29 (74.4) |
10 (25.6) |
26 (66.7) |
13 (33.3) | |||
|
stage |
N0 |
56 (90.3) |
6 (9.7) |
0.039* |
54 (87.1) |
8 (12.9) |
0.024* |
|
N1 |
25 (73.5) |
9 (26.5) |
22 (64.7) |
12 (35.3) | |||
|
N2a |
6 (85.7) |
1 (14.3) |
6 (85.7) |
1 (14.3) | |||
|
N2b |
2 (50.0) |
2 (50.0) |
2 (50.0) |
2 (50.0) | |||
|
TNM stage |
I |
5 (83.3) |
1 (16.7) |
0.049* |
5 (83.3) |
1 (16.7) |
0.036* |
|
II |
51 (91.1) |
5 (8.9) |
49 (87.5) |
7 (12.5) | |||
|
III |
33 (73.3) |
12 (26.7) |
30 (66.7) |
15 (33.3) | |||
|
Tumor budding |
Low-grade |
56 (98.2) |
1 (1.8) |
<0.001# |
54 (94.7) |
3 (5.3) |
<0.001# |
|
Intermediate-grade |
24 (80.0) |
6 (20.0) |
24 (80.0) |
6 (20.0) | |||
|
High-grade |
9 (45.0) |
11 (55.0) |
6 (30.0) |
14 (70.0) | |||
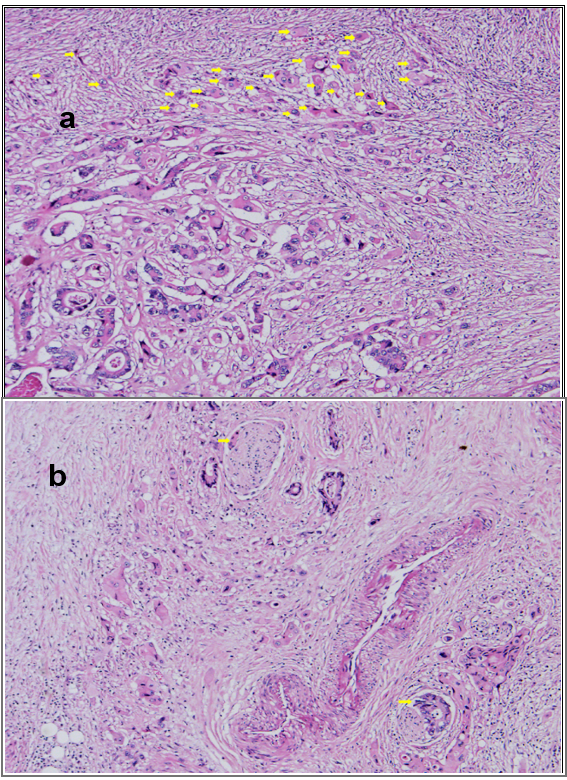
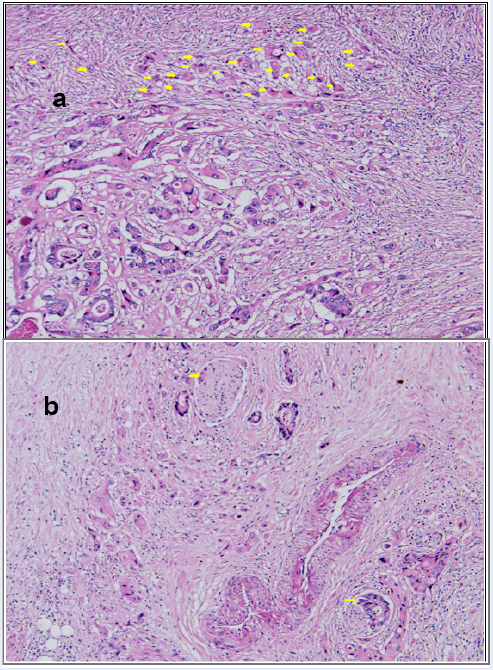
High-grade tumor budding (arrows) (a) and perineural invasion (arrows) (b) (H&E, x200).
Overall and disease-free survival by tumor budding grade
|
Tumor budding |
Cases of deaths due to CRC |
Mean OS |
p-value |
Cases of recurrences |
Mean DFS |
p-value |
|---|---|---|---|---|---|---|
|
Low-grade |
1 |
84.255±0.739 |
<0.001 |
3 |
82.271±1.625 |
<0.001 |
|
Intermediate-grade |
6 |
80.167±4.530 |
6 |
76.8±5.617 | ||
|
High-grade |
11 |
56.1±6.645 |
14 |
42.05±6.843 |
Multivariate Cox regression analysis of some characteristics according to OS and DFS
|
Characteristics |
OS |
DFS | ||
|
HR |
p |
HR |
p | |
|
High-grade |
45.952 |
<0.001 |
20.652 |
<0.001 |
|
Tumor differentiation |
1.67 |
0.433 |
1.293 |
0.682 |
|
Lymphovascular invasion |
1.039 |
0.98 |
1.081 |
0.951 |
|
Perineural invasion |
0.52 |
0.268 |
0.555 |
0.285 |
|
T stage |
1.274 |
0.504 |
1.294 |
0.432 |
|
N stage |
1.466 |
0.412 |
1.189 |
0.69 |
|
TNM stage |
1.021 |
0.989 |
1.361 |
0.808 |
Discussion
CRC is the most prevalent malignancy of the gastrointestinal tract and has a high mortality rate. As reported by GLOBOCAN 2020, the number of new CRC cases worldwide is predicted to reach 3.2 million by 2040, based on aging, population growth, and human development projections1, 8, 9. In Vietnam, CRC had the fifth highest incidence rate, with 16,426 cases reported, following liver, lung, stomach, and breast cancers1.
Predicting survival outcomes in patients with colorectal adenocarcinoma remains a significant challenge, as traditional histopathological factors such as histologic type, grade, and pT stage do not always provide reliable prognostic value. In particular, histological subtypes such as signet-ring cell carcinoma and mixed adenocarcinoma are often associated with worse prognoses, making histopathology-based assessments less accurate. An increasing number of studies have confirmed that gene expression profiles and molecular characteristics offer higher prognostic value than classical histopathological factors. However, the application of molecular biology tests still faces numerous barriers, especially in developing countries such as Vietnam, due to complex technical requirements, high costs, and limited implementation in pathology laboratories. In contrast, Bd assessment using H&E-stained slides does not incur additional costs and can be easily implemented in pathology laboratories. Moreover, a standardized Bd assessment protocol based on the 2016 ITBCC recommendations has been developed, ensuring consistency and high reliability. Bd can also be evaluated consistently by different pathologists, a crucial factor that supports its use as a valuable prognostic indicator.
Bd is defined as the presence of single tumor cells or small clusters of tumor cells (< 5 cells) scattered throughout the tumor stroma. Bd is primarily assessed at the invasive tumor front; however, it has also been described within the tumor center. Lugli . reported that intratumoral budding is strongly associated with peritumoral budding and is an independent prognostic factor5. Bd is linked to the epithelial-mesenchymal transition (EMT), a process involving the loss of cell adhesion, cytoskeletal alterations, and increased extracellular matrix production5, 10.
When evaluating the relationship between Bd and histopathological tumor characteristics, we found that high-grade Bd was more prevalent in patients with deeper invasion, lymphovascular invasion, perineural invasion, and lymph node metastases (p < 0.05). Similarly, Dawson . reported that high-grade Bd was associated with tumor differentiation, invasion, lymph node metastasis, as well as lymphovascular and perineural invasion (p < 0.001)11. Shah . also identified Bd as a significant risk factor for lymph node metastasis, suggesting that it could help predict the extent of lymph node dissection. Specifically, high-grade Bd was observed in 79% of N1-stage cases and 95% of N2-stage cases12. Additionally, Brototo . demonstrated that Bd in preoperative biopsies predicts lymph node metastasis in colon carcinoma13. These findings suggest that the presence of Bd may indicate tumor invasion and spread. Therefore, assessing Bd on H&E-stained slides is essential for guiding treatment decisions and estimating patient prognosis.
A deeper analysis of OS and DFS across different Bd groups revealed that patients with high-grade Bd had significantly lower OS and DFS than those with low-grade Bd. In multivariate analysis, Bd was identified as an independent prognostic factor in CRC. High-grade Bd was also associated with higher risks of recurrence and mortality (p < 0.001), with HRs of mortality and recurrence of 45.952 and 20.652, respectively. Significant differences in OS and DFS among Bd groups were also demonstrated in both univariate and multivariate analyses (p < 0.001). Moreover, among stage II patients, high-grade Bd was associated with reduced DFS rates (57.1%, 85.7%, and 100% for high-, intermediate-, and low-grade Bd, respectively) (p = 0.001). Similarly, high-grade Bd correlated with a higher risk of recurrence, with a mean DFS time of 82.985 months for low-grade tumors, 70.8 months for intermediate-grade tumors, and 42.05 months for high-grade tumors (p < 0.001). Finally, multivariate analysis showed that high-grade Bd is an independent prognostic factor for increased recurrence risk (HR = 33.136, p < 0.01). These findings are consistent with those of previous studies. For example, Van Wyk . observed that high-grade Bd was strongly associated with disease stage, lymphovascular invasion, and decreased DFS, and was an independent prognostic indicator for patient survival irrespective of tumor stage14. Additionally, Shah observed that patients with high-grade Bd frequently experienced earlier recurrence and more extensive metastasis than other patients12. Moreover, a 2016 meta-analysis conducted by Rogers ., which included 34 studies with a total of 7,821 patients, demonstrated that Bd was a strong predictor of lymph node metastasis, recurrence, and mortality15. In addition, Betge . demonstrated that Bd was an independent predictor of disease progression (HR: 3.91, p = 0.02) and cancer-related mortality (HR: 5.9, p = 0.007)16. Lee . also found a correlation between Bd and survival (p < 0.05), suggesting that stage II CRC patient survival could be further stratified by Bd17. Finally, in a study of 200 stage II and 226 stage III CRC cases, Nakamura et al. showed that cumulative 5- and 10-year survival rates were significantly different between patients with low- and high-grade Bd (93.9% . 73.9% and 90.6% . 67.8%, respectively). Moreover, survival rates did not differ significantly between stage II patients with high-grade Bd and those with stage III disease. Cox regression analysis confirmed that Bd was an independent prognostic factor (HR = 4.89; p < 0.001)18.
Bd at the invasive front has been recognized by the Union for International Cancer Control as an unfavorable parameter and an “additional prognostic marker”19. Moreover, high-grade Bd is consistently associated with lymph node metastasis, distant metastasis, local recurrence, and the extent of invasion beyond the muscularis mucosae. Furthermore, Bd has been proposed as a useful predictor of micrometastasis in patients with node-negative CRC20 and as a key consideration for local resection in patients with T1 tumors21, 22. Multiple groups have examined the independent impact of Bd on treatment outcomes and prognosis in CRC. Some studies have shown that Bd is associated with a higher T stage5, 14, 15, 23, lymph node metastasis5, 14, 15, 23, 24, and adverse histological features independent of disease stage5, 14, 20, 23. High-grade Bd also has an independent adverse impact on both OS and DFS5, 14, 15, 16, 17, 20, 22, 23. The adverse prognostic impact of high-grade Bd is observed in both early-stage and advanced CRC and can significantly influence clinical decision-making, especially in early-stage disease. Moreover, even among patients with stage I, stage II, or node-positive disease, Bd has been shown to improve patient risk stratification16, 18, 25, 26. Taken together, Bd is considered relevant in three scenarios: (1) determining the risk of lymph node metastasis in those with early-stage CRC, thereby predicting the need for lymph node dissection; (2) identifying high-risk stage II patients requiring adjuvant therapy; and (3) its presence in the pretreatment biopsy specimen is predictive of metastasis and non-response to neoadjuvant therapy.
While molecular biomarkers such as microsatellite instability (MSI), KRAS, and BRAF mutations have demonstrated significant prognostic value in CRC, their application in routine clinical settings remains limited in many low- and middle-income countries due to high cost and lack of adequate infrastructure. In contrast, Bd can be assessed using standard H&E staining and has been widely endorsed by international guidelines for its reproducibility and prognostic relevance.
Our decision to focus on Bd rather than molecular markers was driven by both practical and clinical considerations. First, the retrospective design and resource-limited setting precluded comprehensive molecular profiling. Second, Bd offers a feasible, cost-effective, and widely applicable approach to risk stratification, especially in stage II CRC, where treatment decisions remain challenging. Third, existing literature has shown that Bd is an independent predictor of adverse outcomes, even when molecular features are accounted for. Therefore, Bd may serve as a surrogate or complementary marker to molecular assays, particularly in settings where access to advanced molecular diagnostics is restricted.
Tumor budding can be readily integrated into routine clinical practice, particularly in resource-limited environments by adhering to standardized assessment protocols. Using only H&E-stained surgical specimens, pathologists can evaluate Bd without the need for additional equipment or specialized reagents. This allows for its inclusion in routine histopathology reports, thereby enhancing the prognostic utility of these reports. Notably, Bd assessment may offer critical insights for guiding therapeutic decisions, especially in stage II CRC patients, where the indication for adjuvant chemotherapy remains a subject of clinical uncertainty.
Our findings are in line with previous studies from resource-limited settings in developing countries, which consistently demonstrate that tumor budding serves as an independent prognostic factor27, 28. These results further support its clinical utility in guiding treatment strategies. In developing countries, many cancer patients cannot afford expensive molecular tests. Since tumor budding analysis is based only on H&E-stained tissue sections, Bd may be a promising candidate for risk stratification but has received little attention in Vietnam. To our knowledge, the current study is the first in Vietnam to apply the ITBCC 2016 Bd classification to predict survival outcomes of CRC patients.
The current study has certain limitations, such as the heterogeneity of the study sample in terms of both TNM stage and treatment regimen. However, the use of the Cox proportional hazards model and multivariate regression analysis to identify factors independently associated with OS and DFS may partially reduce potential biases. Potential confounders such as comorbidities, molecular markers (., MSI status), treatment, and lifestyle factors were not controlled for. Future studies should aim to include these variables to better isolate the prognostic impact of tumor budding. Due to the retrospective and single-center design, selection bias cannot be excluded. Future prospective multicenter validation studies with larger sample sizes are necessary to confirm our findings.
Conclusions
In the present study, Bd was closely associated with survival outcomes, lymph node metastasis, and TNM stage; therefore, it can serve as an independent predictor of survival in patients with CRC. These results provide further evidence supporting Bd’s value as an additional histopathological feature in predicting prognosis in patients with CRC. Given its specific assessment criteria, technical simplicity, and ease of application, Bd has the potential to be widely applied to various types of cancer in developing countries.
Abbreviations
AJCC: American Joint Committee on Cancer, Bd: Tumor budding, CRC: Colorectal cancer, (DFS) Disease-free survival, H&E: hematoxylin and eosin, ITBCC: International Tumor Budding Consensus Conference, OS: Overall survival.
Acknowledgments
We would like to thank the Hanoi Medical University for the study’s approval.
Author’s contributions
DTL, NVH: Conceptualization, Methodology, Writing-Original draft preparation; NVC, DTY: Visualization, Methodology, Software; NTQ, TNN: Data curation, Writing-Original draft preparation; NKD, NVC: Validation, investigation, Supervision. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
None.
Ethics approval and consent to participate
This study was approved by the Ethics Review Board of Hanoi Medical University (IRB-VN 922) and conducted in accordance with the ethical principles of the 1975 Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study, patient anonymity, and the absence of any additional interventions.
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, etc) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

