LncRNA-NONHSAT192404.1 Regulates miR-518a-3p to Inhibit Triple-Negative Breast Cancer Progression via the PI3K/AKT Pathway
- Department of Breast, Chongqing Hospital of Traditional Chinese Medicine, Chongqing 400021, China
- Department of Oncology, Chongqing Hospital of Traditional Chinese Medicine, Chongqing 400021, China
Abstract
Introduction: Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer lacking targeted therapies. This study aimed to elucidate the role of the long non-coding RNA (lncRNA) NONHSAT192404.1 in TNBC progression and its regulatory relationship with miR-518a-3p and the PI3K/AKT signaling pathway.
Methods: Quantitative real-time PCR (qPCR) was used to analyze NONHSAT192404.1 expression in TNBC cell lines. Functional assays, including CCK-8 proliferation, wound-healing, Transwell invasion, flow cytometry, and apoptosis analyses, were performed following NONHSAT192404.1 overexpression. The direct interaction between NONHSAT192404.1 and miR-518a-3p was confirmed using dual-luciferase and RNA pull-down assays. Western blot and qPCR analyses were employed to investigate the regulation of the PI3K/AKT pathway.
Results: NONHSAT192404.1 was significantly down-regulated in TNBC cell lines. Its overexpression inhibited cell proliferation, migration, and invasion, and induced cell-cycle arrest and apoptosis. A direct molecular interaction between NONHSAT192404.1 and miR-518a-3p was established. Furthermore, NONHSAT192404.1 was shown to negatively regulate the PI3K/AKT pathway in a miR-518a-3p-dependent manner.
Conclusion: These findings highlight NONHSAT192404.1 as a potential tumor suppressor in TNBC and suggest that the NONHSAT192404.1/miR-518a-3p/PI3K/AKT axis may serve as a promising therapeutic target.
Introduction
Breast cancer is the most prevalent malignancy among women worldwide, representing a significant public health challenge due to its heterogeneity and variable clinical outcomes1, 2. Among the various subtypes of breast cancer, Triple-Negative Breast Cancer (TNBC) is particularly notorious for its aggressive behavior and poor prognosis3, 4. TNBC is defined by the absence of estrogen receptor (ER), progesterone receptor (PR), and HER2 protein expression, which renders conventional endocrine therapies and HER2-targeted treatments ineffective5. This leaves TNBC patients with limited therapeutic options, typically relying on surgery, radiation, and chemotherapy, which often result in high recurrence rates and poor overall survival6, 7.
Given the urgent need for effective targeted therapies in TNBC, there has been increasing interest in the molecular mechanisms that drive TNBC tumorigenesis8. Studies indicate that long non-coding RNAs (lncRNAs) and circular RNAs are not only involved in TNBC tumorigenesis but also show potential as targets for personalized treatment, offering opportunities for improved monitoring of recurrence and resistance9. Recent advances in genomics and transcriptomics have revealed that lncRNAs play critical roles in cancer biology10. LncRNAs, which are transcripts longer than 200 nucleotides that do not encode proteins, have been shown to regulate gene expression at various levels, including chromatin remodeling, transcriptional control, and posttranscriptional processing11. The specificity of lncRNA expression profiles makes them promising for early diagnosis and targeted treatment in TNBC12. Their dysregulation is associated with various oncogenic processes, including cell proliferation, invasion, and metastasis1, 13, 14. Studies indicate that lncRNAs like LINC01087 and NRAD1 show promise as diagnostic biomarkers and therapeutic targets in TNBC due to their differential expression and potential roles in cancer progression5, 15, 16. Additionally, research highlights that lncRNAs interact with miRNAs in TNBC, regulating gene expression and progression, thus presenting potential targets for neoadjuvant chemotherapy17, 18. Among these, miR-518a-3p is a microRNA recognized for its tumor-suppressive properties19, but its interaction with lncRNAs and specific impact on TNBC cell behavior remain poorly understood.
The PI3K/AKT pathway is a critical regulator of cell survival, growth, and metabolism and is frequently activated in various cancers, including TNBC20, 21. Dysregulation of this pathway is common in TNBC and plays a central role in promoting cancer cell survival and chemoresistance, making it a key molecular target for developing effective therapies22, 23. Research has shown that approximately 25–30 % of TNBC cases feature genetic alterations in the PI3K/AKT/mTOR pathway, suggesting that targeted therapies may be particularly beneficial for these patients24. Specific mutations in PIK3CA and other pathway components have been associated with TNBC progression, indicating the importance of the pathway in this cancer subtype25. Recent findings suggest that certain long non-coding RNAs (lncRNAs) may activate the PI3K/AKT pathway in TNBC, providing potential new targets for therapeutic intervention26. Recent studies have shown that lncRNAs can regulate the PI3K/AKT signaling pathway by acting as molecular sponges for specific miRNAs27. LncRNA H19 promotes PI3K/AKT activation by binding to miR-140-5p in ovarian cancer, while PTENP1 enhances PTEN expression by sponging miR-17 and miR-19b, leading to pathway suppression27, 28, 29. These findings support the idea that lncRNA–miRNA interactions play a key role in modulating PI3K/AKT activity in cancer.
LncRNA-NONHSAT192404.1 is a newly identified lncRNA whose role in TNBC has yet to be fully elucidated30. Here, we identified lncRNA-NONHSAT192404.1 as significantly upregulated in TNBC tissues and cell lines, suggesting its potential role in the pathogenesis of this aggressive cancer subtype. Mechanistically, we found that lncRNA-NONHSAT192404.1 interacts with miR-518a-3p to modulate gene expression post-transcriptionally, with subsequent effects on the PI3K/AKT signaling pathway. This study aims to investigate the function and molecular mechanism of lncRNA-NONHSAT192404.1 in TNBC, focusing on its interaction with miR-518a-3p and its downstream regulation of the PI3K/AKT signaling pathway. Our findings are expected to contribute to identifying novel biomarkers and potential therapeutic targets in TNBC.
Methods
Cell Culture
Triple-negative breast cancer (TNBC) cell lines MDA-MB-231 (ATCC, HTB-26) and the non-tumorigenic mammary epithelial cell line Hs578Bst (ATCC, HTB-126) were obtained from the American Type Culture Collection (ATCC, USA). TNBC cells and MDA-MB-231 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM; Gibco, Thermo Fisher Scientific, Catalog No. 11965092) supplemented with 10 % fetal bovine serum (FBS; Gibco, Catalog No. 10082147), 1 % penicillin-streptomycin (Gibco, Catalog No. 15140122), and 2 mM L-glutamine (Gibco, Catalog No. 25030081). All cell lines were maintained at 37 °C in a humidified atmosphere containing 5 % CO. Cells were passaged at 70–80 % confluence using 0.25 % trypsin-EDTA (Gibco, Catalog No. 25200072).
LncRNA and miR-518a-3p Overexpression and Knockdown
For overexpression of LncRNA-NONHSAT192404.1 and miR-518a-3p, full-length sequences of LncRNA-NONHSAT192404.1 and precursor miR-518a-3p were cloned into the pcDNA3.1(+) vector (Invitrogen, Thermo Fisher Scientific, Catalog No. V79020). For knockdown experiments, small interfering RNAs (siRNAs) targeting LncRNA-NONHSAT192404.1 and antisense oligonucleotides (ASOs) targeting miR-518a-3p were synthesized by GenePharma (Shanghai, China). Cells were transfected with overexpression constructs, siRNAs, or ASOs using Lipofectamine 3000 (Invitrogen, Thermo Fisher Scientific, Catalog No. L3000015) according to the manufacturer’s protocol. The sequences of the siRNAs used for knockdown are listed in Supplementary Table 1.
Transfection efficiency was confirmed by quantitative real-time PCR (qRT-PCR) 48 h post-transfection. Scrambled siRNA or ASO was used as a negative control.
Quantitative Real-Time PCR (qRT-PCR)
Expression levels of LncRNA-NONHSAT192404.1, NONHSAT148701.1, and miR-518a-3p were measured in TNBC cell lines (MDA-MB-231) and compared with the normal mammary epithelial cell line (Hs578Bst). Total RNA was extracted using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Catalog No. 15596026). miRNA was extracted with the mirVana miRNA Isolation Kit without phenol (Thermo Fisher Scientific, AM1561). RNA concentration and purity were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Catalog No. ND-2000). cDNA was synthesized from 1 µg total RNA using the PrimeScript RT Reagent Kit (Takara Bio, Japan, Catalog No. RR037A). qRT-PCR was performed with SYBR Green Master Mix (Applied Biosystems, Thermo Fisher Scientific, Catalog No. 4309155) on a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems, Catalog No. 4485690). Expression levels of LncRNA-NONHSAT192404.1, miR-518a-3p, and other genes of interest were normalized to GAPDH or U6 snRNA, respectively, and calculated by the 2 method. Primer sequences are provided in Supplementary Table 2.
Cell Proliferation Assay
Cell proliferation was assessed with the Cell Counting Kit-8 (CCK-8) assay (Dojindo Laboratories, Japan, Catalog No. CK04-11). Cells were seeded in 96-well plates at 2,000 cells/well in 100 µL complete medium. After overnight adhesion they were transfected as described. At 24, 48, 72, and 96 h post-transfection, 10 µL CCK-8 solution was added per well and incubated for 2 h at 37 °C. Absorbance was read at 450 nm on a microplate reader (Bio-Rad, USA, Catalog No. 168-1000EDU). Experiments were done in triplicate.
Cell Cycle Analysis
Cell-cycle distribution was analyzed by flow cytometry after propidium iodide (PI) staining. Briefly, MDA-MB-231 cells (transfected as indicated) were harvested 48 h post-transfection, washed twice with ice-cold PBS, and fixed in 70 % ethanol at 4 °C overnight. After washing, cells were incubated with PI (50 µg/mL) and RNase A (100 µg/mL) in PBS for 30 min at room temperature in the dark, and DNA content was measured on a flow cytometer. Percentages of cells in G₀/G₁, S, and G₂/M phases were determined with appropriate software.
Apoptosis Detection
Apoptotic cells were quantified by Annexin V-FITC/PI dual staining followed by flow cytometry. Forty-eight hours after transfection, MDA-MB-231 cells were harvested by trypsinization, washed twice with PBS, and resuspended in 100 µL 1× binding buffer. Annexin V-FITC (5 µL) and PI (5 µL) from the Annexin V-FITC/PI Apoptosis Detection Kit (KeyGEN BioTECH, China; Cat. No. KGA107) were added and incubated for 10 min at room temperature in the dark. Samples were analyzed within 1 h. Cells were classified as viable (Annexin V⁻/PI), early apoptotic (Annexin V⁺/PI), and late apoptotic/necrotic (Annexin V⁺/PI).
Cell Invasion Assay
The invasive capacity of TNBC cells was evaluated in Transwell chambers (Corning, USA, Catalog No. 3422) with 8-µm-pore polycarbonate membranes coated with Matrigel (BD Biosciences, USA, Catalog No. 354234) diluted 1:8 in serum-free DMEM. Hs578Bst was included as a negative control. Cells (5 × 10⁴) in serum-free medium were seeded in the upper chamber; DMEM with 10 % FBS was placed in the lower chamber as chemoattractant. After 24 h, non-invading cells were removed, and invading cells were fixed with 4 % paraformaldehyde, stained with 0.1 % crystal violet, and counted under a microscope (Olympus, Japan, Catalog No. CX23) in five random fields. Experiments were done in triplicate.
Wound-Healing Assay
Transfected TNBC cells were seeded at 5 × 10⁵ cells/well in 6-well plates and grown to 90 % confluence. A linear scratch was made with a 200 µL pipette tip, debris was removed with PBS (Gibco, Thermo Fisher Scientific, Catalog No. 10010049), and cells were cultured in serum-free DMEM. Images were captured at 0, 24, and 48 h with an inverted microscope. Wound width was measured in ImageJ and quantified as previously described31.
Luciferase Reporter Assay
To validate the interaction between LncRNA-NONHSAT192404.1 and miR-518a-3p, the pmirGLO dual-luciferase vector (Promega, USA; Cat. No. E1330) was used. A fragment of the TLR4 3′-UTR containing the predicted miR-518a-3p site was cloned downstream of firefly luciferase; a mutant site was generated with the QuikChange II kit (Agilent Technologies, USA; Cat. No. 200523). MDA-MB-231 cells were co-transfected with wild-type, mutant, or empty pmirGLO plus miR-518a-3p mimics (GenePharma, China) using Lipofectamine 3000. After 48 h, luciferase activity was measured with the Dual-Luciferase Reporter Assay System (Promega, USA; Cat. No. E1910). Experiments were done in triplicate.
RNA Pull-Down Assay
Direct interaction between LncRNA-NONHSAT192404.1 and miR-518a-3p was tested by a biotin-labeled RNA pull-down assay. Wild-type and mutant transcripts of LncRNA-NONHSAT192404.1 were transcribed with 3′-biotin and incubated with streptavidin-magnetic beads using the Pierce Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher Scientific, USA; Cat. No. PI20164). After incubation with MDA-MB-231 lysates, bound RNA was eluted and miR-518a-3p enrichment quantified by qRT-PCR. Scrambled biotin-RNA served as negative control.
Western Blot
MDA-MB-231 cells were transfected as indicated. Total protein was extracted with RIPA buffer (Cell Signaling Technology, USA, Catalog No. 9806S) plus protease/phosphatase inhibitors (Thermo Fisher Scientific, Catalog No. 78440). Protein concentration was determined with the BCA kit (Thermo Fisher Scientific, Catalog No. 23225). Equal protein amounts (30 µg) were resolved by SDS-PAGE on 4–20 % gradient gels (Bio-Rad, USA, Catalog No. 4561094) and transferred by wet transfer to PVDF membranes (Millipore, USA, Catalog No. IPVH00010). Membranes were blocked with 5 % non-fat milk in TBST (Cell Signaling Technology, Catalog No. 9997S) for 1 h and incubated overnight at 4 °C with primary antibodies (1:1000): TLR4 (Proteintech, 19811-1-AP), AKT (Proteintech, 10176-2-AP), PI3K (Proteintech, 27921-1-AP), PPP2R1A (Proteintech, 15882-1-AP), and GAPDH (Proteintech, 10494-1-AP). After washing, membranes were incubated with HRP-conjugated secondary antibodies (Cell Signaling Technology, Catalog No. 7074S; 1:2000) for 1 h at room temperature. Bands were visualized with ECL substrate (Bio-Rad, Catalog No. 1705061) and imaged on a ChemiDoc XRS+ system (Bio-Rad, USA, Catalog No. 1708265). Densitometry was performed with Image Lab.
Bioinformatics Analysis
Potential miR-518a-3p target genes were predicted with TargetScanHuman 7.2. Functional enrichment (GO terms, KEGG pathways) was analyzed in DAVID v6.8. Protein-protein interaction networks were generated with STRING v11.0 and visualized in Cytoscape v3.
Statistics
Data are presented as mean ± SD. Two-group comparisons used Student’s t-test; multiple groups used one-way ANOVA with Tukey’s post-hoc test. p < 0.05 was considered significant. Analyses were performed in GraphPad Prism.
Results
Downregulation of LncRNA-NONHSAT192404.1 and NONHSAT148701.1 in TNBC Cells
To evaluate the expression levels of LncRNA-NONHSAT192404.1 and NONHSAT148701.1 in triple-negative breast cancer (TNBC), we conducted quantitative real-time PCR (qRT-PCR) analyses in the TNBC cell line MDA-MB-231 and compared them with normal mammary epithelial cells (Hs578Bst). The results revealed a significant downregulation of LncRNA-NONHSAT192404.1 in TNBC cells relative to Hs578Bst cells (Figure 1A). Similarly, LncRNA-NONHSAT148701.1 was found to be significantly downregulated in TNBC cells (Supplementary Fig. 1A). These findings imply a potential involvement of LncRNA-NONHSAT192404.1 and NONHSAT148701.1 in the oncogenic processes driving TNBC. We then analyzed survival data from TNBC patients. Kaplan–Meier survival curves were generated to assess the relationship between LncRNA expression levels and patient prognosis. The analysis revealed a significant positive correlation between the expression levels of LncRNA-NONHSAT192404.1 and patient survival, indicating that higher levels of this LncRNA are associated with better outcomes in TNBC patients (Figure 1B). Similarly, increased expression of LncRNA-NONHSAT148701.1 was also found to be positively correlated with patient survival, further supporting its potential role as a prognostic biomarker in TNBC (Supplementary Fig. 1B).
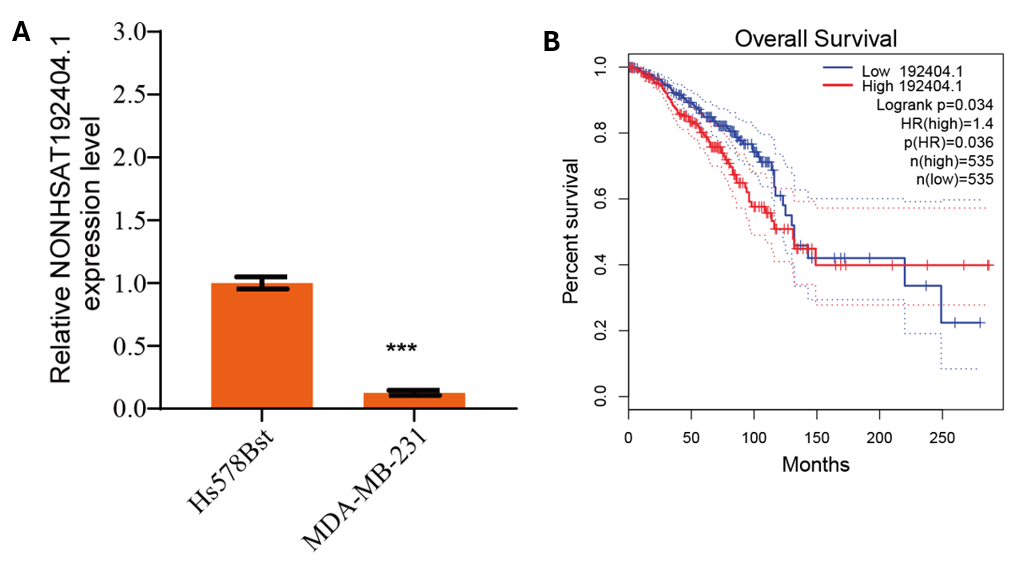
Expression levels of LncRNA-NONHSAT192404.1, NONHSAT148701.1, and miR-518a-3p in TNBC cells and their association with patient prognosis. (A) qRT-PCR analysis of lncRNA-NONHSAT192404.1, NONHSAT148701.1, and miR-518a-3p expression in TNBC cell line (MDA-MB-231) compared to normal mammary epithelial cells (Hs578Bst). NONHSAT192404.1 and NONHSAT148701.1 were significantly downregulated, whereas miR-518a-3p was upregulated in TNBC cells. (n = 3). Error bars represent mean ± SEM. ***p < 0.001. (B) Kaplan-Meier survival analysis showing better overall survival in TNBC patients with high NONHSAT192404.1 expression. (C) Kaplan-Meier survival analysis showing better overall survival in TNBC patients with high NONHSAT148701.1 expression.
LncRNA-NONHSAT192404.1 Inhibits TNBC Cell Proliferation and Induces Apoptosis
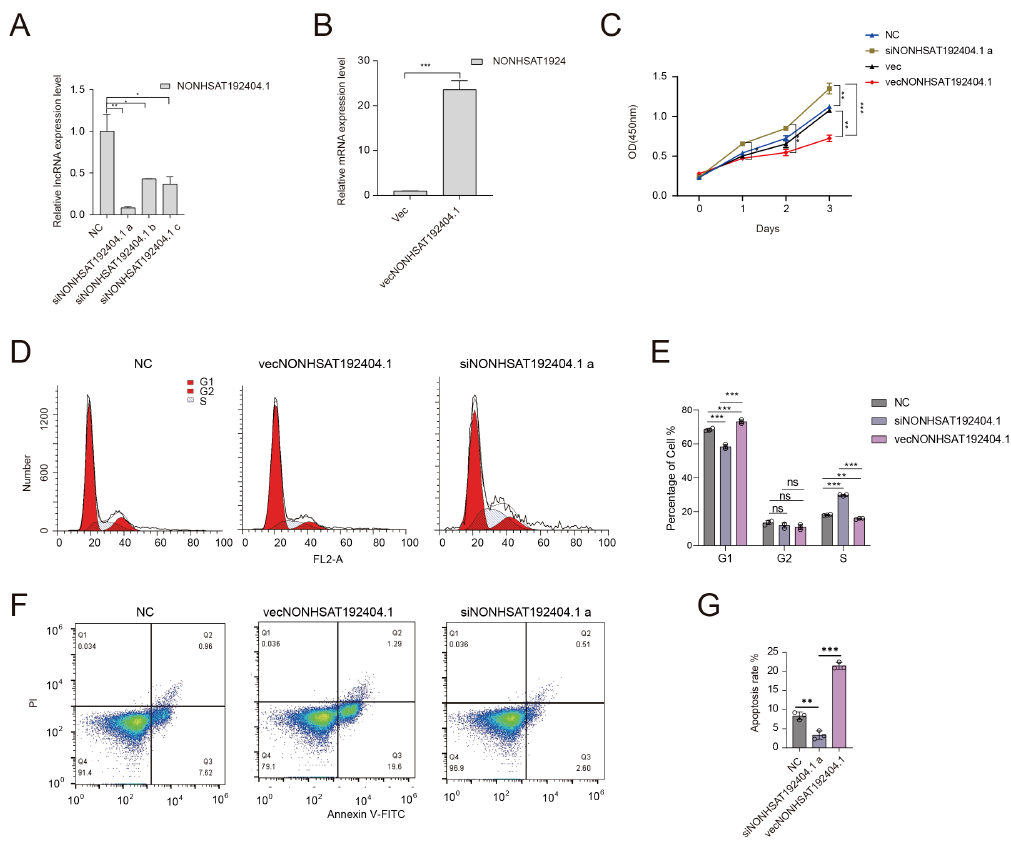
The influence of LncRNA-NONHSAT192404.1 on TNBC cell proliferation was evaluated using the Cell Counting Kit-8 (CCK-8) assay. Overexpression of LncRNA-NONHSAT192404.1 in MDA-MB-231 cells led to a significant decrease in proliferation (Figure 2A-C). In contrast, knock-down of LncRNA-NONHSAT192404.1 resulted in a marked increase in cell proliferation (Figure 2A-C), indicating that LncRNA-NONHSAT192404.1 plays a crucial role in inhibiting TNBC-cell proliferation. To understand the mechanistic basis of LncRNA-NONHSAT192404.1’s effects, we examined its impact on cell-cycle progression. Flow-cytometry analysis revealed that overexpression of LncRNA-NONHSAT192404.1 led to an increase in the proportion of cells in the S phase, with a concomitant decrease in the M phase, suggesting cell-cycle arrest at S (Figure 2D, E). In addition, overexpression significantly increased apoptosis, as evidenced by a higher percentage of Annexin-V-positive cells, whereas knock-down reduced apoptosis rates (Figure 2F, G).
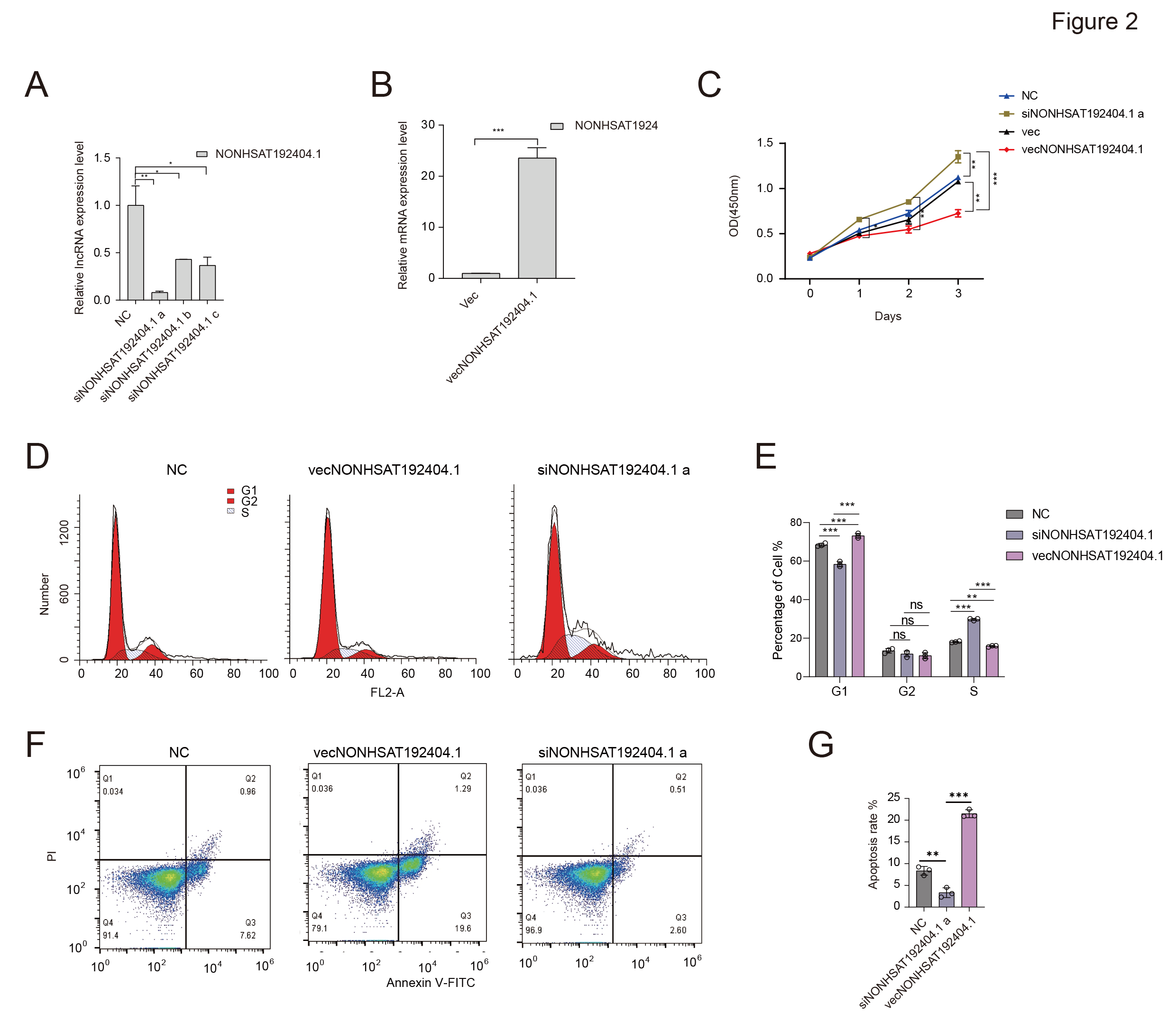
LncRNA-NONHSAT192404.1 Inhibits TNBC Cell Proliferation and Induces Apoptosis. (A-C) Overexpression of lncRNA-NONHSAT192404.1 significantly decreases TNBC cell proliferation, while its knockdown enhances proliferation as shown by the CCK-8 assay. (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001. (D, E) Flow cytometry analysis shows that lncRNA-NONHSAT192404.1 overexpression leads to cell cycle arrest at the S phase, while knockdown decreases S phase cells. (n = 3). p < 0.05. (F, G) Apoptosis analysis shows that lncRNA-NONHSAT192404.1 overexpression increases apoptotic cell death, while its knockdown reduces apoptosis. (n = 3). Error bars represent mean ± SEM. **p < 0.01, ***p < 0.001.
LncRNA-NONHSAT192404.1 Suppresses TNBC Cell Migration and Invasion
The wound-healing assay further supported these findings, demonstrating that cells overexpressing LncRNA-NONHSAT192404.1 exhibited slower wound closure, while knock-down significantly increased wound-closure ability, suggesting a role in cell migration (Figure 3A, B). To further investigate invasion, Transwell assays were performed. Overexpression of LncRNA-NONHSAT192404.1 significantly inhibited invasion of MDA-MB-231 cells. Conversely, knock-down resulted in a significant elevation in cell invasion (Figure 3C, D).
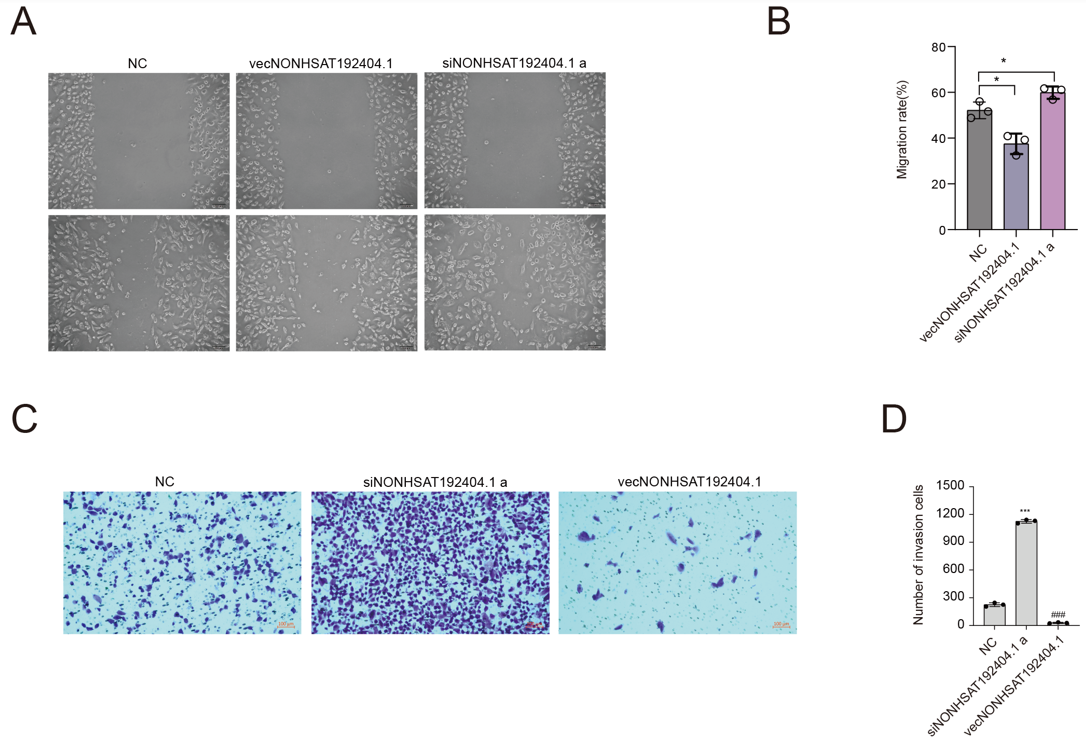
LncRNA-NONHSAT192404.1 Suppresses TNBC Cell Migration and Invasion. (A, B) Wound healing assay demonstrates that overexpression of lncRNA-NONHSAT192404.1 inhibits cell migration, whereas knockdown promotes faster wound closure. Scale bar = 100 µm. (n = 3). *p < 0.05. (C, D) Transwell invasion assay reveals that lncRNA-NONHSAT192404.1 overexpression reduces the invasive capacity of TNBC cells, while knockdown increases invasion. Scale bar = 50 µm. (n = 3). ***p < 0.001.
LncRNA-NONHSAT192404.1 Directly Interacts with miR-518a-3p
Based on bioinformatics predictions using TargetScan, which identified a putative binding site for miR-518a-3p within LncRNA-NONHSAT192404.1, we hypothesized a direct interaction (Figure 4A). miR-518a-3p was significantly up-regulated in MDA-MB-231 relative to normal mammary epithelial cells (Figure 4B). Dual-luciferase assays showed a significant reduction in luciferase activity. Importantly, mutating the miR-518a-3p binding site abolished this reduction, confirming direct binding (Figure 4C). An RNA pull-down assay corroborated these findings: miR-518a-3p was specifically enriched in complexes with LncRNA-NONHSAT192404.1 but not with control RNA (Figure 4D, E). These results demonstrate that LncRNA-NONHSAT192404.1 directly interacts with, and likely sponges, miR-518a-3p in TNBC cells.
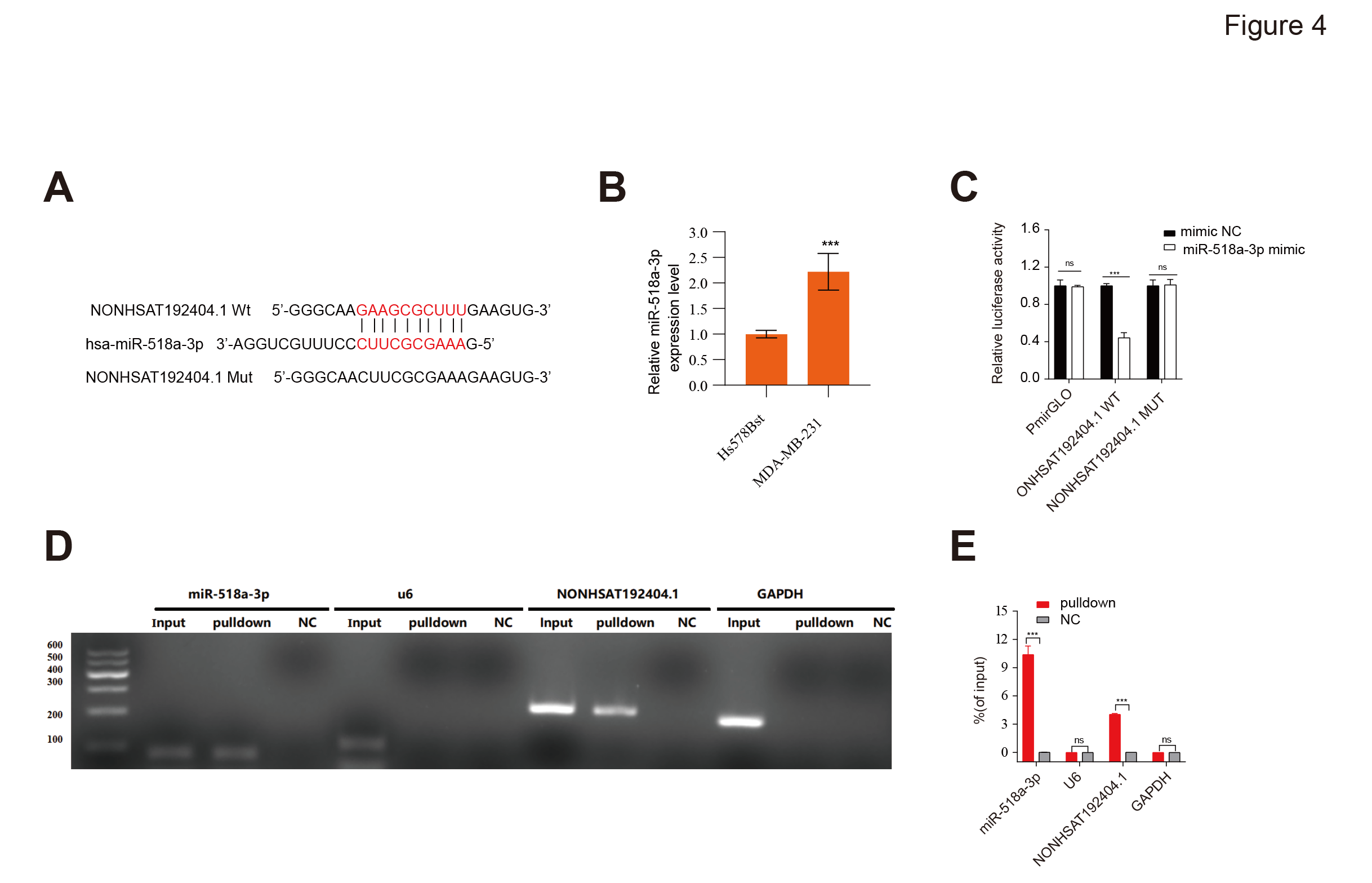
Direct interaction between LncRNA-NONHSAT192404.1 and miR-518a-3p. (A) Schematic representation of the predicted miR-518a-3p binding site within the sequence of NONHSAT192404.1. The wild-type and mutant binding site sequences used for luciferase reporter construction are shown. (B) Luciferase reporter assay confirms direct binding between NONHSAT192404.1 and miR-518a-3p. Mutations in the miR-518a-3p binding site of lncRNA-NONHSAT192404.1 abolish this interaction. (n = 3). Error bars represent mean ± SEM. ***p < 0.001.(C,D) RNA pulldown assay further validates the direct interaction between lncRNA-NONHSAT192404.1 and miR-518a-3p in TNBC cells. (n = 3). Error bars represent mean ± SEM. * **p < 0.001.
Regulation of PI3K/AKT Signaling by LncRNA-NONHSAT192404.1 Is Reversible by miR-518a-3p
To explore how LncRNA-NONHSAT192404.1 regulates PI3K/AKT signaling, we quantified TLR4, AKT, PI3K and PPP2R1A expression. PI3K, AKT and PPP2R1A were significantly up-regulated in MDA-MB-231 versus Hs578Bst cells, whereas TLR4 was down-regulated (Figure 5A). Western-blotting showed that overexpression of LncRNA-NONHSAT192404.1 increased TLR4 and decreased AKT, PI3K and PPP2R1A (Fig. 5B). Notably, miR-518a-3p expression reversed the PI3K/AKT activation induced by LncRNA-NONHSAT192404.1 (Figure 5B).
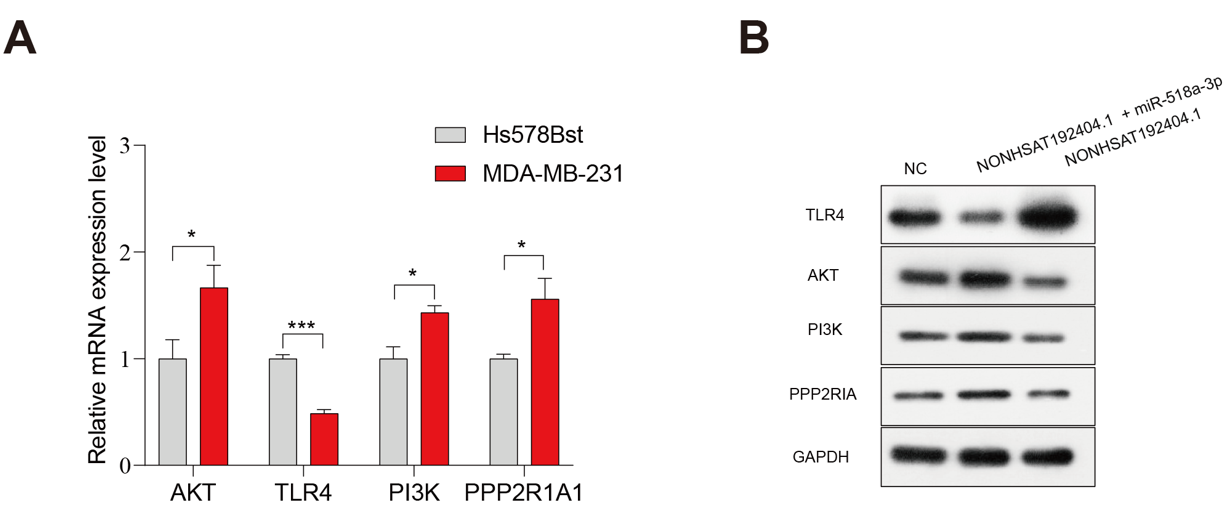
Regulation of the PI3K/AKT signaling pathway by LncRNA-NONHSAT192404.1 is reversible by miR-518a-3p. (A) qPCR analysis of PI3K, TLR4, AKT, and PPP2R1A expression in MDA-MB-231 cells relative to normal mammary epithelial cells (Hs578Bst, used as control). (n = 3). *p < 0.05, ***p < 0.001. (B) Western blot analysis showing the protein levels of PI3K, AKT, TLR4, and PPP2R1A in MDA-MB-231 cells following overexpression or knockdown of lncRNA-NONHSAT192404.1. Co-transfection with miR-518a-3p mimics reversed the effects of lncRNA overexpression, while miR-518a-3p inhibitors reversed the effects of lncRNA knockdown. GAPDH was used as a loading control.
Discussion
In this study, we provide compelling evidence that lncRNA-NONHSAT192404.1 and NONHSAT148701.1 are significantly down-regulated in TNBC cells compared with normal mammary epithelial cells. Our data reveal that lncRNA-NONHSAT192404.1 inhibits TNBC cell proliferation, migration, and invasion. Over-expression of this lncRNA suppresses these oncogenic behaviors, whereas its knock-down enhances them, indicating its critical role in TNBC progression. Furthermore, our mechanistic studies suggest that lncRNA-NONHSAT192404.1 exerts its effects, at least in part, by modulating the PI3K/AKT signaling pathway, a well-known pathway implicated in cancer progression.
We identified a direct interaction between lncRNA-NONHSAT192404.1 and miR-518a-3p. Our luciferase reporter and RNA pull-down assays demonstrate that lncRNA-NONHSAT192404.1 can act as a molecular sponge for miR-518a-3p, thereby regulating its availability and activity. This interaction may represent a novel regulatory mechanism by which lncRNA-NONHSAT192404.1 influences TNBC cell behavior.
LncRNA-NONHSAT192404.1 and NONHSAT148701.1 emerge as potential therapeutic targets in TNBC. Targeting these lncRNAs could offer a new strategy to inhibit TNBC cell proliferation and invasion, thereby improving patient outcomes. Future studies should focus on the in-vivo validation of these lncRNAs as therapeutic targets and further exploration of their roles in TNBC pathogenesis.
Our findings align with the growing body of literature describing lncRNA–miRNA crosstalk in regulation of the PI3K/AKT pathway across cancers. For example, several studies have reported that lncRNAs acting as competitive endogenous RNAs (ceRNAs) regulate PI3K/AKT by sequestering oncogenic miRNAs32. The identification of miR-518a-3p as a functional target of NONHSAT192404.1 adds a novel node to this regulatory network specifically in TNBC. While many ceRNA models involve lncRNAs sequestering miRNAs that target PI3K/AKT pathway members27, our data extend this paradigm by showing that NONHSAT192404.1 itself is regulated by PI3K/AKT-related mechanisms via TLR4. Interestingly, some other TNBC-associated lncRNAs modulate different miRNAs or act through alternate pathways, which may reflect context-dependent expression profiles and binding-site availability, highlighting the importance of cell-specific investigations.
In our study, over-expression of NONHSAT192404.1 led to increased TLR4 expression but reduced PI3K/AKT pathway activity, which seems inconsistent with the classical view that TLR4 activates PI3K/AKT via the MyD88 adaptor to promote cell growth and survival. However, recent studies suggest that PI3K/AKT may also negatively regulate TLR4 signaling. For example, activated AKT can inhibit FOXO1, a transcription factor that promotes TLR4 expression, forming a feedback loop. In macrophages, PI3K/AKT also limits TLR4/NF-κB–mediated inflammation, indicating a complex interaction33, 34. In cancer, although TLR4 over-expression often enhances NF-κB activity and tumor progression, PI3K/AKT signaling can still be suppressed by other mechanisms, such as miRNA targeting or PTEN activation. This suggests that high TLR4 expression does not always lead to increased AKT activity35, 36, 37, 38. Our results support this complexity: the relationship between TLR4 and PI3K/AKT is not strictly linear but is influenced by multiple layers of regulation.
Although the downstream targets of miR-518a-3p within the PI3K/AKT pathway have not yet been definitively characterized, recent evidence suggests that miR-518a-3p may regulate the PI3K/AKT pathway by targeting key components such as PI3K subunits, AKT isoforms, or upstream modulators like PIK3IP1. While the exact targets of miR-518a-3p remain to be fully identified, studies on similar miRNAs (., miR-19a-3p and miR-520a-3p) have shown direct inhibition of PI3K/AKT signaling27. In our system, it is likely that miR-518a-3p also suppresses PI3K/AKT activity by binding to relevant pathway molecules. In future studies, we will further investigate the downstream targets of miR-518a-3p in the PI3K/AKT pathway.
Our findings indicate that restoring NONHSAT192404.1 or inhibiting miR-518a-3p may suppress PI3K/AKT-mediated tumor progression in TNBC, highlighting NONHSAT192404.1 as a potential biomarker or therapeutic target. However, this study was limited to the MDA-MB-231 cell line, and further validation in additional TNBC models and patient samples is necessary. Future research should also assess its in-vivo relevance, examine downstream signaling events such as phosphorylation, and explore upstream regulatory mechanisms to better understand its role in TNBC pathogenesis.
Conclusions
Our study characterizes a novel regulatory mechanism by which lncRNA-NONHSAT192404.1 attenuates TNBC aggressiveness through interaction with miR-518a-3p and modulation of PI3K/AKT signaling. These findings contribute to a deeper understanding of noncoding RNA regulation in TNBC and provide a foundation for translational research targeting this axis.
Abbreviations
ASO: Antisense Oligonucleotide, ATCC: American Type Culture Collection, BCA: Bicinchoninic Acid, cDNA: Complementary DNA, CCK-8: Cell Counting Kit-8, ceRNA: Competitive Endogenous RNA, CO₂: Carbon Dioxide, DAVID: Database for Annotation, Visualization and Integrated Discovery, DMEM: Dulbecco's Modified Eagle Medium, ECL: Enhanced Chemiluminescence, EDTA: Ethylenediaminetetraacetic acid, ER: Estrogen Receptor, FBS: Fetal Bovine Serum, FITC: Fluorescein Isothiocyanate, FOXO1: Forkhead Box O1, GAPDH: Glyceraldehyde 3-Phosphate Dehydrogenase, GO: Gene Ontology, HER2: Human Epidermal Growth Factor Receptor 2, HRP: Horseradish Peroxidase, KEGG: Kyoto Encyclopedia of Genes and Genomes, lncRNA: Long Non-Coding RNA, miRNA/miR: MicroRNA, mTOR: Mammalian Target of Rapamycin, MyD88: Myeloid Differentiation Primary Response 88, NF-κB: Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells, PBS: Phosphate-Buffered Saline, PI: Propidium Iodide, PI3K: Phosphoinositide 3-Kinase, PIK3CA: Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha, PIK3IP1: Phosphoinositide-3-Kinase Interacting Protein 1, PR: Progesterone Receptor, PPP2R1A: Protein Phosphatase 2 Scaffold Subunit Aalpha, PTEN: Phosphatase and Tensin Homolog, PVDF: Polyvinylidene Fluoride, qPCR/qRT-PCR: Quantitative Real-Time Polymerase Chain Reaction / Quantitative Reverse Transcription Polymerase Chain Reaction, RIPA: Radioimmunoprecipitation Assay, RNA: Ribonucleic Acid, SD: Standard Deviation, SDS-PAGE: Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis, siRNA: Small Interfering RNA, STRING: Search Tool for the Retrieval of Interacting Genes/Proteins, TBST: Tris-Buffered Saline with Tween 20, TLR4: Toll-Like Receptor 4, TNBC: Triple-Negative Breast Cancer, UTR: Untranslated Region
Acknowledgments
We thank all the members of Gang Lv’s Laboratory for their assistance throughout this research.
Author’s contributions
H.Y. and G.L. was responsible for the study's conception, design, and funding acquisition. L.G. performed the experiments and data analysis. Y.Z. contributed to data interpretation and manuscript revision. J.M. assisted with the experiment setup and provided critical feedback during manuscript preparation. Z.X. and T.W. contributed to the experimental design and data collection. S.H. helped with statistical analysis and manuscript revision. All authors have read and approved the final manuscript.
Funding
This work was supported by the Special Project for Performance Incentives and Guidance of Scientific Research Institutions in Chongqing (cstc2022jxjl120038), the Chongqing Hospital of Traditional Chinese Medicine College “unveiling and commanding” project (JBGS2024-003), the Chongqing Municipal Health Commission Traditional Chinese Medicine Key Discipline Construction Project (YZY [2021] No. 16).
Availability of data and materials
Data is provided within the manuscript or supplementary information files.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Chongqing Hospital of Traditional Chinese Medicine (approval number 2023-ky-52).
Consent for publication
Not applicable.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that they have not used generative AI (a type of artificial intelligence technology that can produce various types of content including text, imagery, audio and synthetic data. Examples include ChatGPT, NovelAI, Jasper AI, Rytr AI, DALL-E, etc) and AI-assisted technologies in the writing process before submission.
Competing interests
The authors declare that they have no competing interests.

